First step is adiabatic (q = 0) so `DeltaU_(1)=w_(1)`
Second step is isochoric (w = 0)
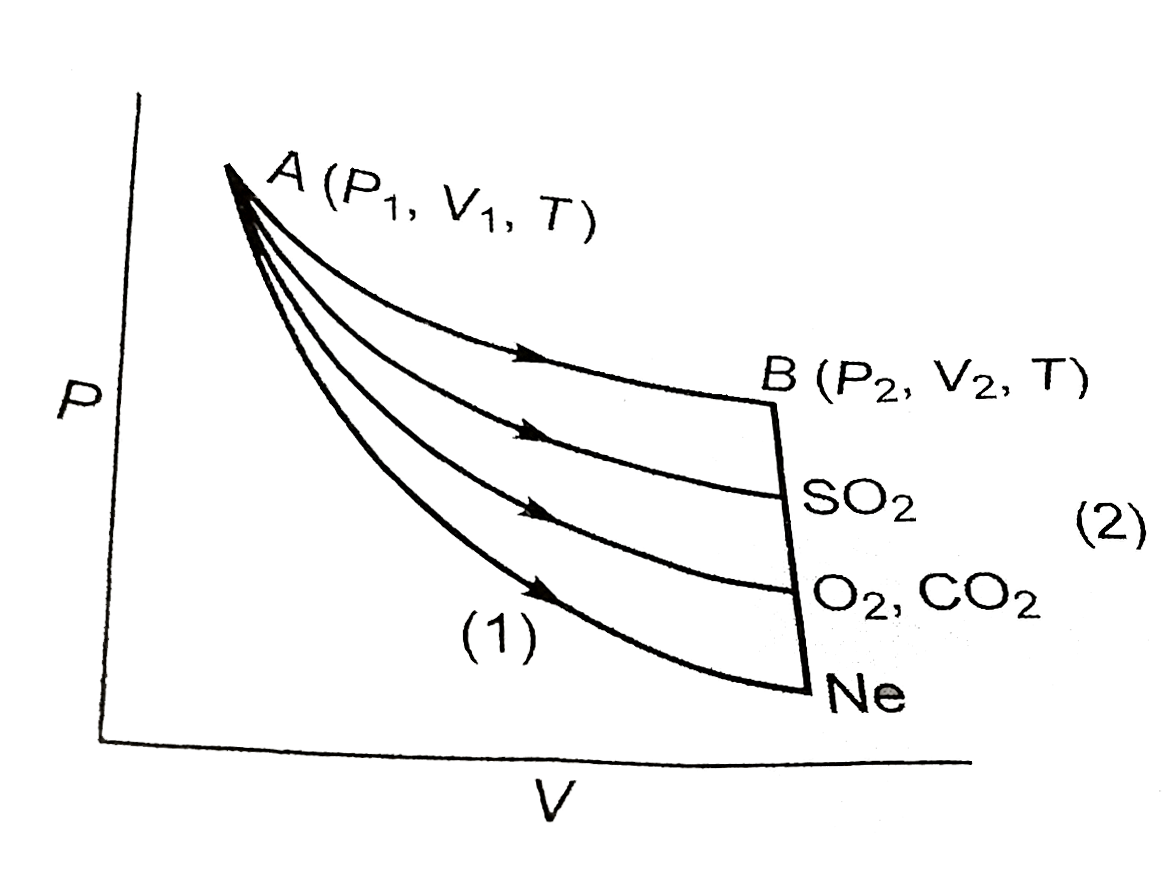
So, `" "DeltaU_(2)=q_(2)`
`because` initial and final temp. are same
`therefore" "DeltaU_("toal")=DeltaU_(1)+DeltaU_(2)=0`
` "or "w_(1)+q_(2)=0`
Max. work done by the gas, `SO_(2)` is (area) under the curve
so, `SO_(2)` absorbed
`because " "gamma_(SO_(2))lt gamma_(CO_(2))=gamma_(O_(2))=ltgamma_(Ne)`
so, max. decrease in temp. of Ne due to step 1.