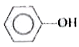
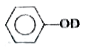
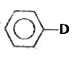
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the given reaction PhMgBr+D(2)O to (X) , (X) will be :
Text Solution
|
- If f(1)(x) and f(2)(x) are defined on domains D(1) and D(2), respectiv...
Text Solution
|
- For the given cell Pt(D(2)|D^(o+))||H^(o+)|Pt(H(2)) , if E^(c-).(D(2)|...
Text Solution
|
- underset((2)H^(o+)//H(2)O)overset((1) PhMgBr)to product (3^(@) alcoh...
Text Solution
|
- If the total number of neutrons present in D(2)O^(18) molecules is x t...
Text Solution
|
- In the given reaction PhMgBr+D(2)Orarr(X),(X) will be :
Text Solution
|
- Cl-overset(O)overset(||)C-Cloverset(PhMgBr(excess))underset(H(3)^(oplu...
Text Solution
|
- Correct rate of reaction with PhMgBr is :
Text Solution
|
- How can D(2)O(2) prepared form water ? Mention the physcial properties...
Text Solution
|