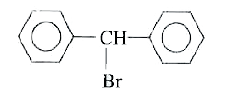
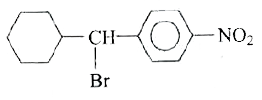
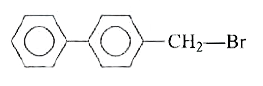
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The rate of Snl reaction is fastest with :
Text Solution
|
- Rate of the reaction. is fastest when Z is
Text Solution
|
- Fastest rate of reaction will be when R is :
Text Solution
|
- Which reaction take place at the fastest rate?
Text Solution
|
- Which reaction occurs at the fastest rate ?
Text Solution
|
- which reaction will occur at the fastest rate?
Text Solution
|
- Which reaction will occur at the fastest rate?
Text Solution
|
- Rate of the reaction is fastest when Z is
Text Solution
|
- Rate of the reaction : is fastest when Z is :
Text Solution
|