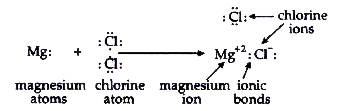Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON-METALS
OSWAL PUBLICATION|Exercise Board corner (short answer type questions - II)|11 VideosMETALS AND NON-METALS
OSWAL PUBLICATION|Exercise Board corner(Long answer type questions)|20 VideosMETALS AND NON-METALS
OSWAL PUBLICATION|Exercise Board corner (very short answer type questions)|1 VideosMETALS AND NON - METALS
OSWAL PUBLICATION|Exercise CASE - BASED MCQs|29 VideosNEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION
OSWAL PUBLICATION|Exercise PERIODIC CLASSIFICATION OF ELEMENTS (VISUAL CASE - BASED QUESTIONS)|15 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-METALS AND NON-METALS-Board corner (short answer type questions)
- (a). Write down the electron arrangement in (i) a magnesium atom, and ...
Text Solution
|
- Gold, platinum and silver are used to make jewellery. Give reasons.
Text Solution
|
- Give reason: Sodium, potassium and lithium are stored under oil
Text Solution
|
- Silver articles become black when kept in open for some time, whereas ...
Text Solution
|
- give reason for the following: Carbonate and sulphide ores are usual...
Text Solution
|
- Give reason: Aluminium is a highly reactive metal, yet it is used to...
Text Solution
|
- Name a metal of medium reactivity and write three main steps in the ex...
Text Solution
|