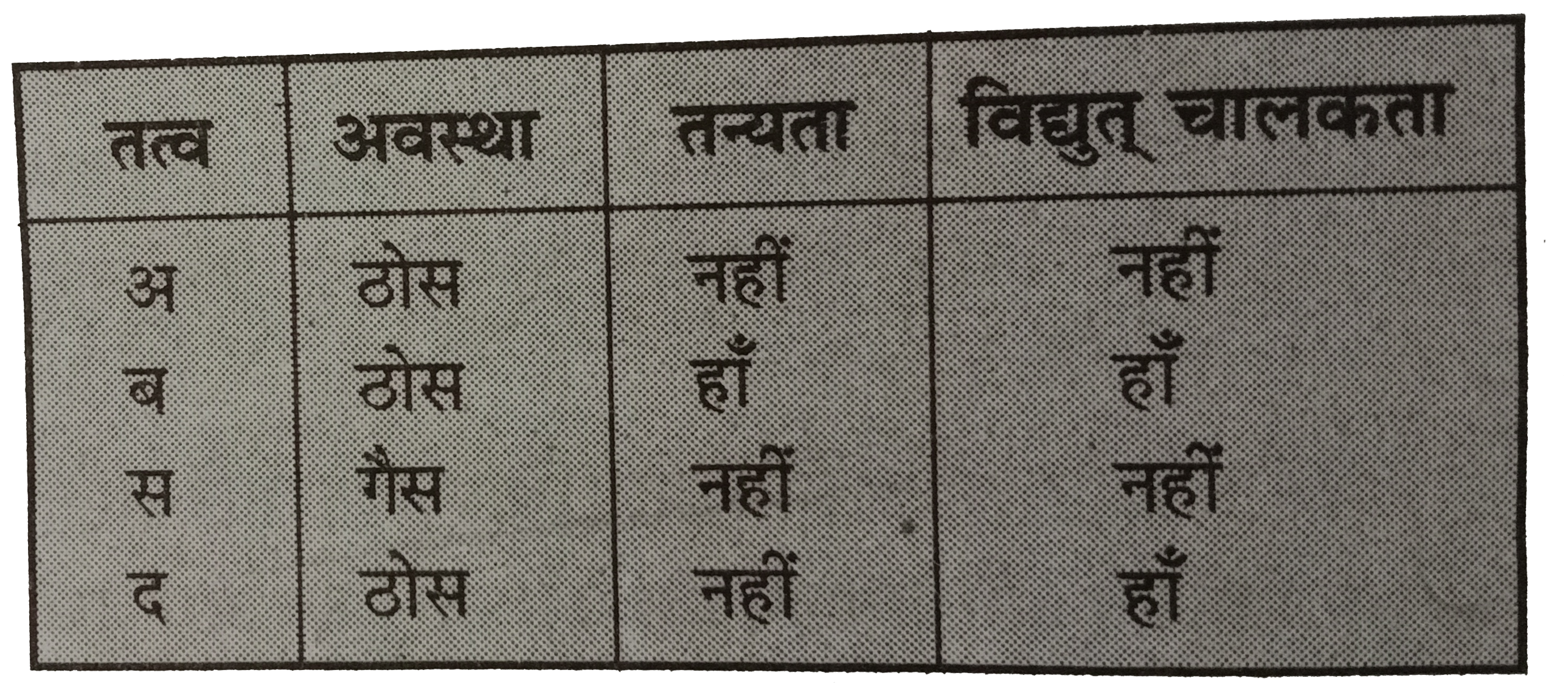
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise EXERCISE (MCQs) LEVEL - 2 |20 VideosPERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise EXERCISE (MATCHING)|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise SOLVED EXAMPLES |10 VideosMATTER IN OUR SURROUNDINGS
MTG IIT JEE FOUNDATION|Exercise OLYMPIAD/HOTS CORNER|15 VideosSTRUCTURE OF THE ATOM
MTG IIT JEE FOUNDATION|Exercise OLYMPIAD / HOTS CORNER |15 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-PERIODIC CLASSIFICATION OF ELEMENTS -EXERCISE (MCQs) LEVEL - 1
- Elements were classified into metals and non-metals by
Text Solution
|
- The scientist who introduced law of triad is
Text Solution
|
- Law of octaves was proposed by
Text Solution
|
- In 1869, Mendeleev formulated a law, which states that the properties ...
Text Solution
|
- Mendeleev predicted the existence of two elements and named them as Ek...
Text Solution
|
- Electronic configurations for four elements A, B, C and D are given be...
Text Solution
|
- The vertical columns in a periodic table are called
Text Solution
|
- Third period of the periodic table contains the following number of el...
Text Solution
|
- Eka-boron predicted by Mendeleev, was named as .......... after it's d...
Text Solution
|
- The first group elements are called
Text Solution
|
- The elements which have incomplete penultimate shell are
Text Solution
|
- Two elements A and B belong to group 1 and 2 respectively. Identify th...
Text Solution
|
- What type of oxide would Eka-aluminium form?
Text Solution
|
- The elements of the group 16 are called .....
Text Solution
|
- The atoms of elements belonging to the same group of periodic table ha...
Text Solution
|
- Which amongst the following is not an alkaline earth metal ?
Text Solution
|
- Which amongst the following is not a noble gas?
Text Solution
|
- From top to bottom in a group of periodic table the electropositive ch...
Text Solution
|
- Arrange the following elements in the order of their increasing atomic...
Text Solution
|
- Which of the following statements about the modern periodic table is c...
Text Solution
|