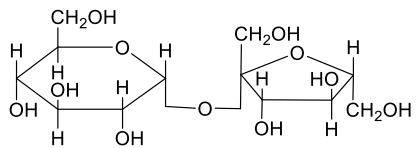
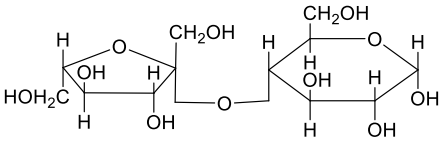
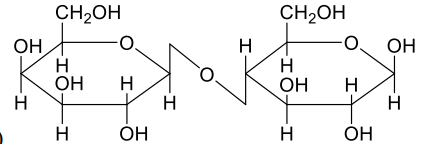
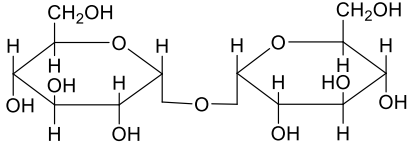
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE ADVANCED PREVIOUS YEAR- MOCK TEST 2022-QUESTIONS
- A disaccharide X cannot be oxidised by bromine water. The acid hydroly...
Text Solution
|
- Which green coloured compound of chromium is formed in borax bead test...
Text Solution
|
- Calamine, malachite, magnetite and cryolite, respectively, are
Text Solution
|
- Molar conductivity (Lamda(m)) of aqueous solution of sodium stearate, ...
Text Solution
|
- A tin chloride Q undergoes the following reactions (not balanced) Q+...
Text Solution
|
- Fusion of MnO2 with KOH in the presence of O2 produces a salt W. Alka...
Text Solution
|
- Choose the reaction, for which the standard enthalpy of reaction is eq...
Text Solution
|
- Which of the following statement(s) is (are) are correct regarding the...
Text Solution
|
- Each of the following options contains a set of four molecules. Identi...
Text Solution
|
- In the decay sequence, {:(238),(92):}Uoverset(-x(1))rarr{:(234),(90)...
Text Solution
|
- Which of the following statement(s) is(are) true?
Text Solution
|
- Among B(2)H(6),B(3)N(3)H(6),N(2)O,N(2)O(4),H(2)S(2)O(3) and H(2)S(2)O(...
Text Solution
|
- For the following reaction, equilibrium constant K(c) at 298 K is 1.6x...
Text Solution
|
- On dissolving 0.5 g of non-volatile, non-ionic solute to 39 g of benze...
Text Solution
|
- Consider the kinetic data given in the following table for the reactio...
Text Solution
|