Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MULTIPLE CHOICE -I)|10 VideosHYDROCARBONS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MULTIPLE CHOICE -II)|9 VideosHYDROCARBONS
PRADEEP|Exercise NCERT QUESTION AND EXERCISES WITH ANSWERS|14 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-NCERT QUESTION AND EXERCISES WITH ANSWERS (NCERT EXCERCISES)
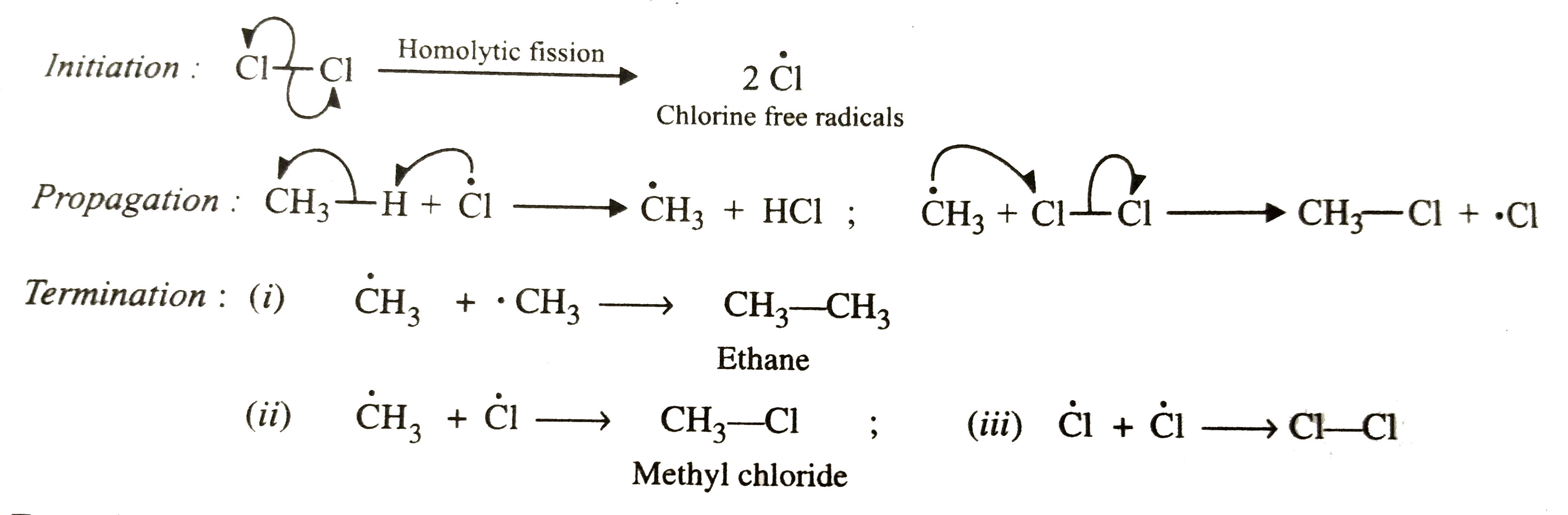
- How do you account for formation of ethane during chlorination of meth...
Text Solution
|
- Write IUPAC names of the following compounds : (a)CH3CH=C(CH3)2 , (b...
Text Solution
|
- For the following compounds, write structural formulas and IUPAC names...
Text Solution
|
- Write IUPAC names of the products obtained by the ozonolysis of the fo...
Text Solution
|
- An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3-on...
Text Solution
|
- An alkene ‘A’ contains three C – C, eight C – H (sigma) bonds and one...
Text Solution
|
- Propanal and pentan-3-one are the ozonolysis products of an alkene? Wh...
Text Solution
|
- Write chemical equations for combustion reaction of the following hydr...
Text Solution
|
- Draw the cis and trans structures of hex-2-ene. Which isomer will have...
Text Solution
|
- Why is benzene extra ordinarily stable though it contains three double...
Text Solution
|
- What are the necessary conditions for any system to be aromatic?
Text Solution
|
- Explain why the following systems are not aromatic ?
Text Solution
|
- How will you convert benzene into (i) p-nitrobromobenzene (ii) m-...
Text Solution
|
- In the alkane H3C-CH(2)-C(CH(3))2-CH(2)-CH(CH(3))2, identify 1^(@),2^(...
Text Solution
|
- What effect does branching of an alkane chain has on its boiling point...
Text Solution
|
- Addition of HBr to propene yields 2-bromopropane, while in the presenc...
Text Solution
|
- Write down the products of ozonolysis of 1, 2-dimethylbenzene (o-xylen...
Text Solution
|
- Arrange benzene, n-hexane and ethyne in decreasing order of acidic beh...
Text Solution
|
- Why does benzene undergo electrophilic substitution reactions easily a...
Text Solution
|
- How would you convert the following compounds into benzene? (i) Ethy...
Text Solution
|