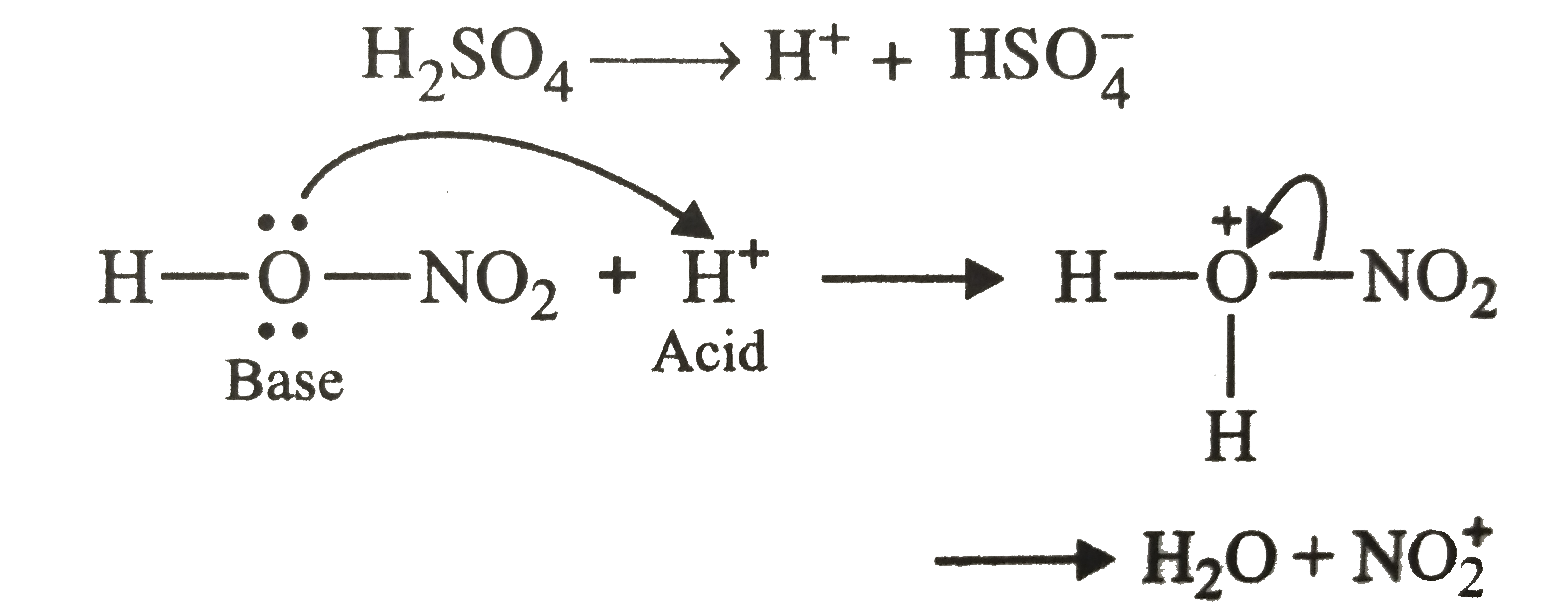
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) II. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) III. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|2 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-Competition Focus (JEE(main and advanced)/Medical Entrance) I. MULTIPLE CHOICE QUESTIONS
- Which of the following compounds will not undergo Friedel- Crafts reac...
Text Solution
|
- The electrophile that participates in nitration of benzene is
Text Solution
|
- Nitrobenzenen can be prepared from benzene by using a mixture of conc ...
Text Solution
|
- The major product(s) obtained from the following reaction of 1 mole of...
Text Solution
|
- Benzene reacts with I2 in presence of which of the following to give i...
Text Solution
|
- Aromatic electrophilic substitution reaction that is reversible is
Text Solution
|
- The compound X in the reaction
Text Solution
|
- Which of the following undergoes nitration most easily?
Text Solution
|
- Among the following compounds the one that is most reactive towards el...
Text Solution
|
- Which of the following has the highest point ?
Text Solution
|
- Chlorobenzene on treatment with sodium in dry ether gives diphenyl. Th...
Text Solution
|
- The following desulphonation reaction, takes place through intermed...
Text Solution
|
- The electrophile, E^(o+) attacks the benzene ring to generate the inte...
Text Solution
|
- 1, 4-Dimethyl benzene on heating with anhydrous AICI(3)" and "HCI prod...
Text Solution
|
- The reaction of toluene with Cl(2) in presence of FeCl(3) gives pre...
Text Solution
|
- Toluene react with a halogen in the presence of iron (III) chloride gi...
Text Solution
|
- Find the major product in the following reaction
Text Solution
|
- Presence of a nitro group in a benzene ring.
Text Solution
|
- Which of the following substituents deactivates the benzene ring towar...
Text Solution
|
- Some meta-directing substituents in aromatic substitution are given wh...
Text Solution
|