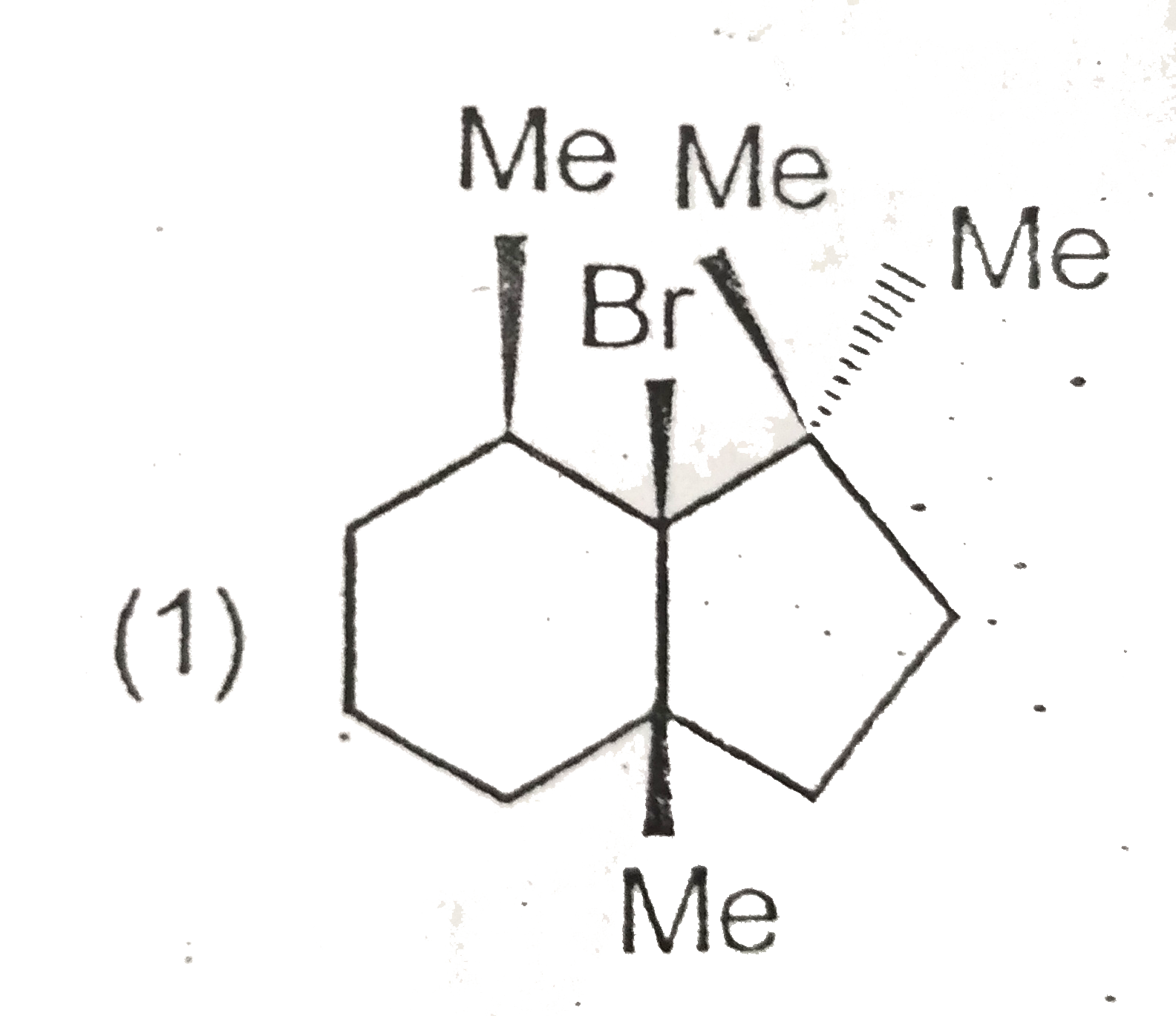Topper's Solved these Questions
HALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise Try Yourself|40 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise EXERCISE|40 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise Assignment (Section - I) (Subjective Type Questions)|10 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE|Exercise Try Yourself|33 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - D) (Assertion -Reason Type Question)|24 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HALOALKANES AND HALOARENES -Assignment (Section - I) (Aakash Challenger Questions)
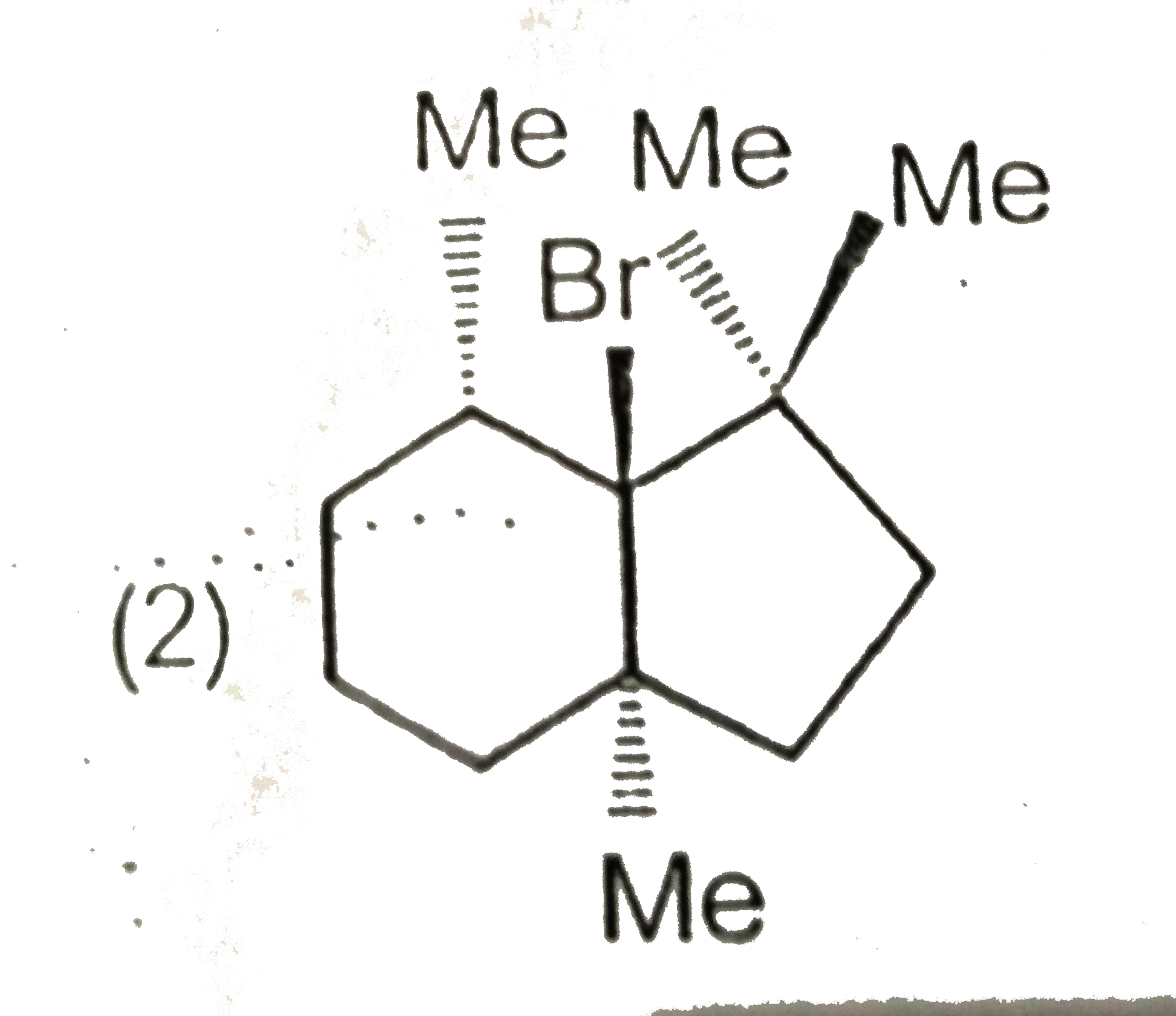
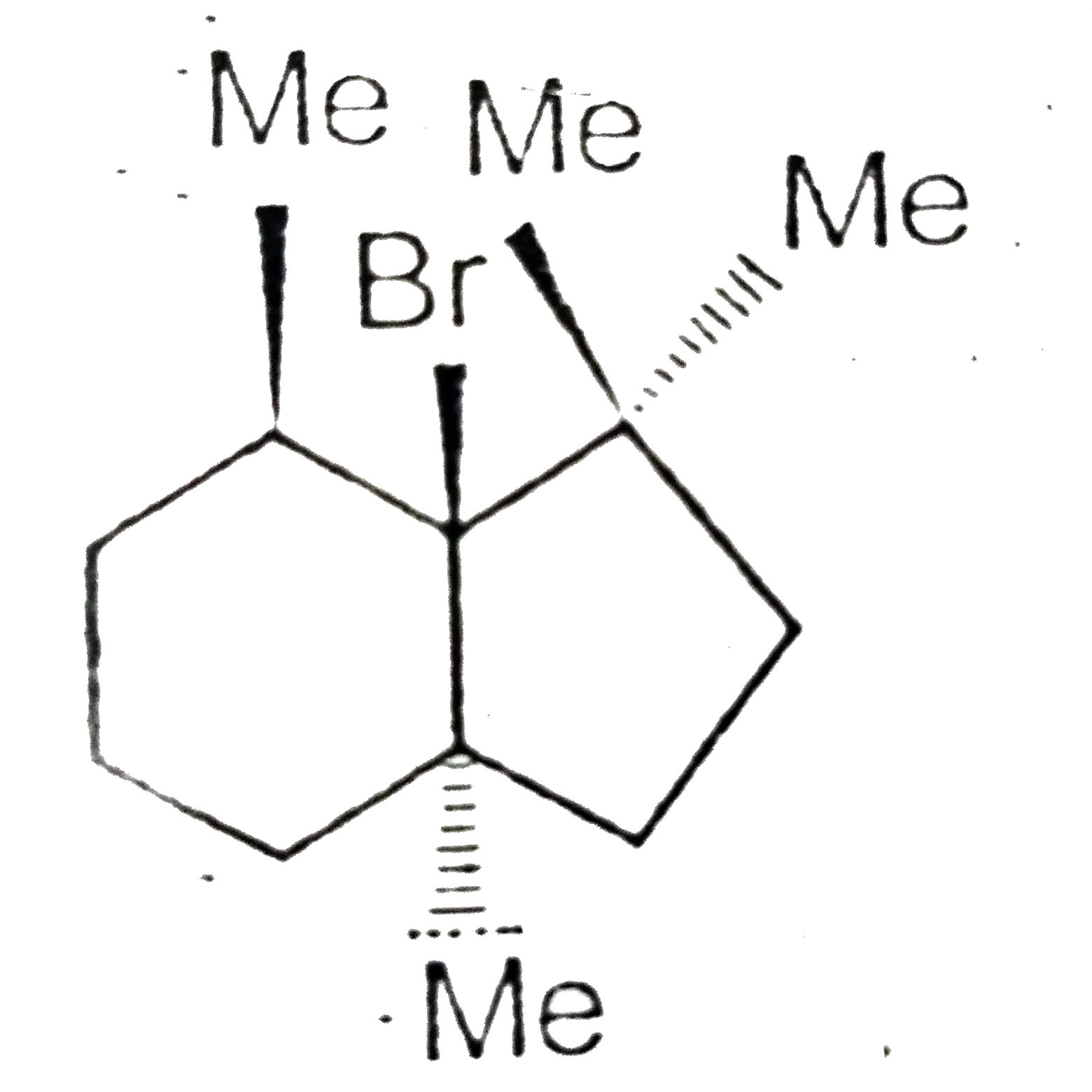
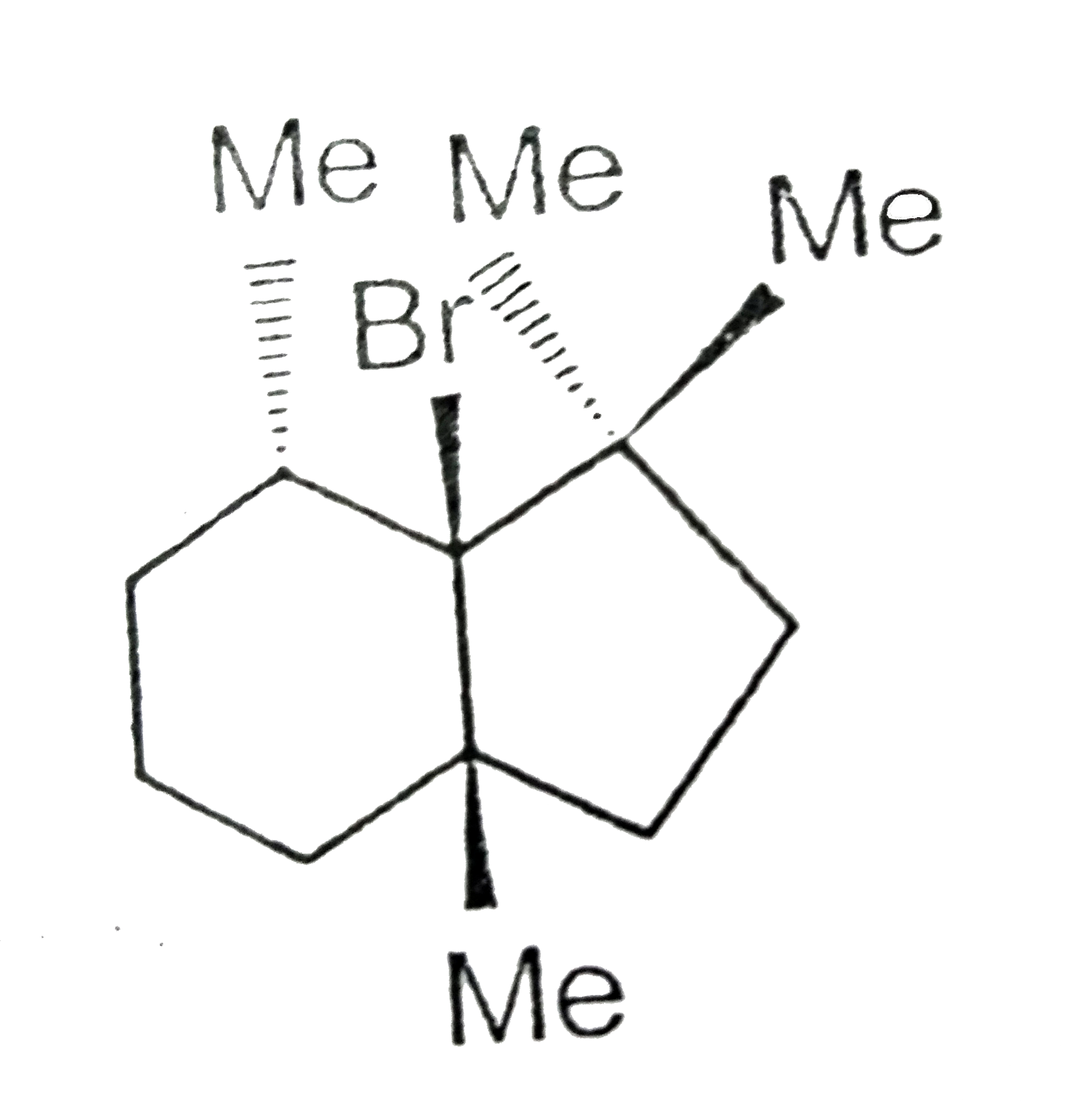
- Which will not react by E(2) mechanism ?
Text Solution
|
- Write the structure of predominant bicycloalkadiene formed in the...
Text Solution
|
- How many elimination products are formed when the given dibrom...
Text Solution
|
- How many mono chloro derivatives are possible , when the given ...
Text Solution
|
- The reagents that will accomplish the following transformation ...
Text Solution
|