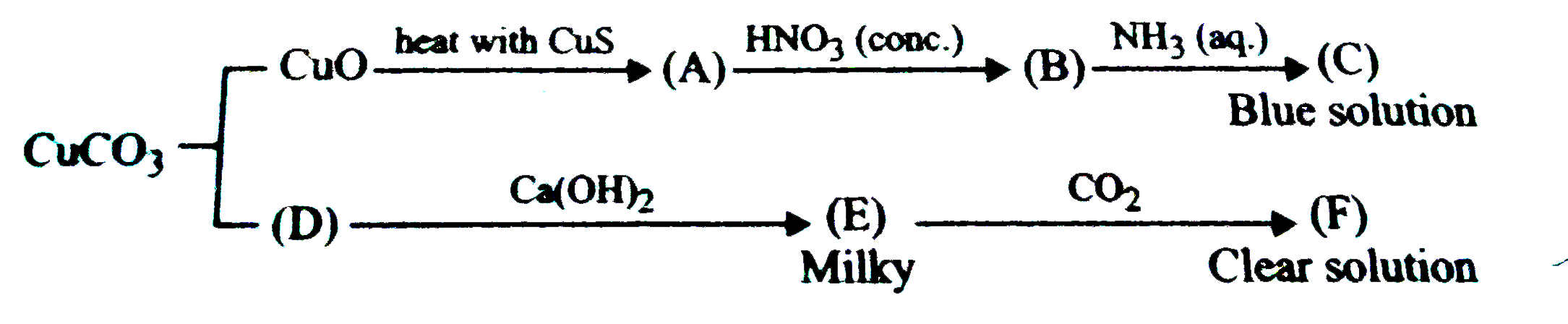Text Solution
Verified by Experts
Topper's Solved these Questions
D- AND F-BLOCK ELEMENTS
PRADEEP|Exercise ADDITIONAL QUESTIONS (VERY SHORT ANSWER QUESTIONS)|49 VideosD- AND F-BLOCK ELEMENTS
PRADEEP|Exercise ADDITIONAL QUESTIONS (SHORT ANSWER QUESTIONS )|34 VideosD- AND F-BLOCK ELEMENTS
PRADEEP|Exercise ASSERTION AND REASON TYPE QUESTIONS|5 VideosCORDINATION COMPOUNDS
PRADEEP|Exercise Important Questions|28 VideosELECTROCHEMISTRY
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|25 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-D- AND F-BLOCK ELEMENTS-LONG ANSWER QUESTIONS
- Identify A to E and also explain the reactions involved.
Text Solution
|
- When a chromite ore (A) is fused with sodium carbonate in free excess ...
Text Solution
|
- When an oxide of manganese (A) is fused with KOH in the presence of an...
Text Solution
|
- On the basis of lanthanoid contraction, explain the following: (i)...
Text Solution
|
- Answer the following questions (i) Which element of the first transi...
Text Solution
|
- Mention the type of compounds formed when small atoms like H, C and N ...
Text Solution
|
- (a) Transition metals can act as catalysts because these can change t...
Text Solution
|
- A violet compound of manganese (A) decomposes on heating to liberate o...
Text Solution
|