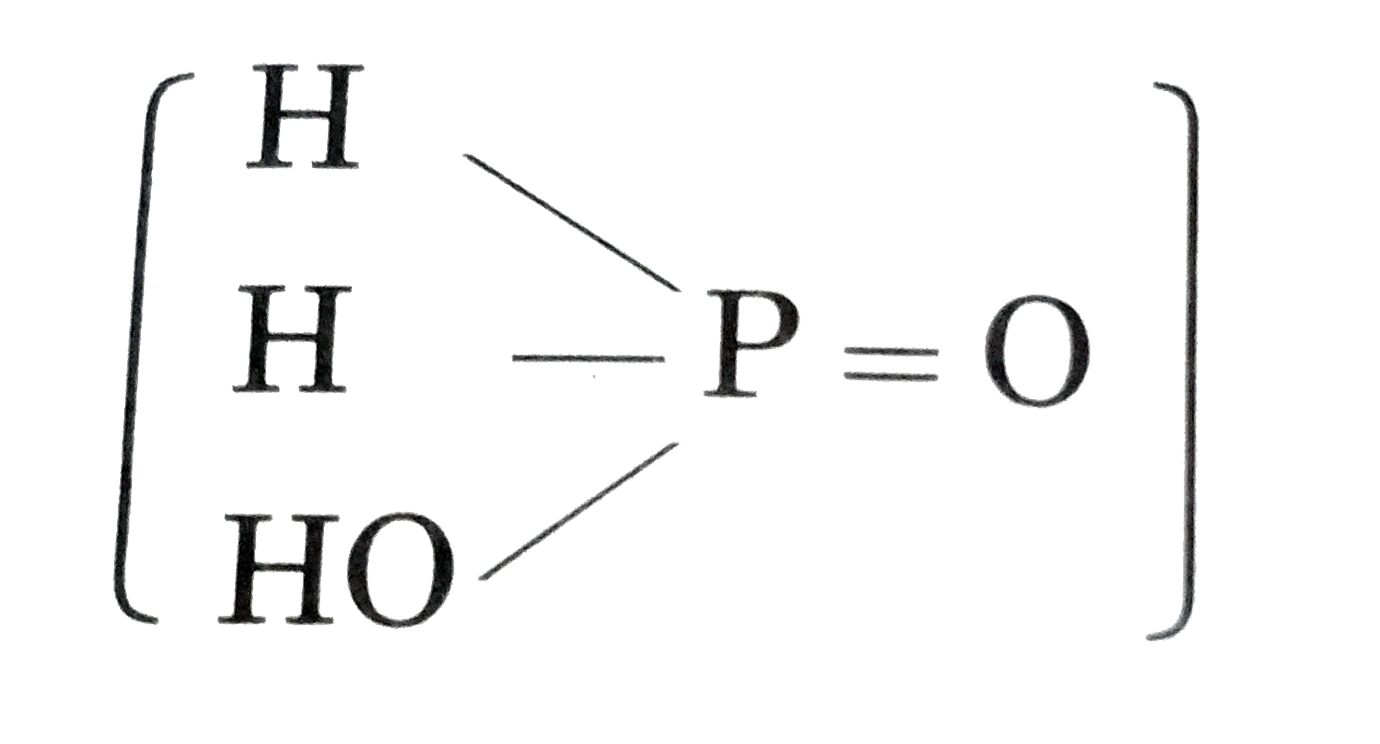Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Why does H(3)PO(2) behave as reducing agent?
Text Solution
|
- Why does H(3)PO(3) act as a reducing agent but H(3)PO(4) does not ?
Text Solution
|
- H(3)PO(2) and H(3)PO(3) act as good reducing agents while H(3)PO(4) do...
Text Solution
|
- Why is H(3)PO(2) a stronger reducing agent than H(3PO(3) ?
Text Solution
|
- Orthophosphoric acid (H(3)PO(4)) is not a reducing agent wheres hypoph...
Text Solution
|
- H(3)PO(2)andH(3)PO(3) act as good reducing agents but H(3)PO(4) does n...
Text Solution
|
- Why does H(3)PO(3) act as a reducing agent but H(3)PO(4) does not ?
Text Solution
|
- Why does H(3)PO(2) behave as reducing agent?
Text Solution
|
- H(3)PO(2) is a good reducing agent - Explain with an example.
Text Solution
|