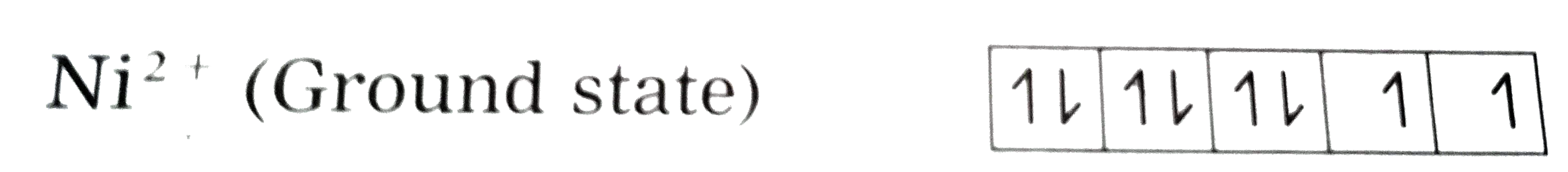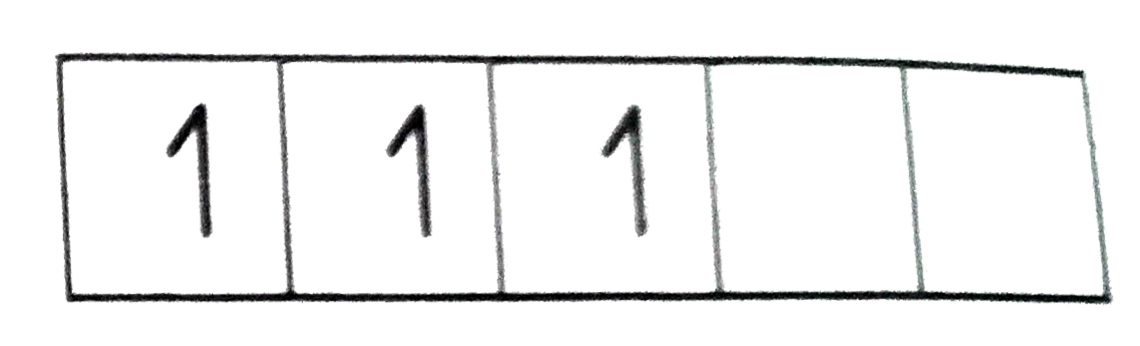Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- [Ni(CN)(4)]^(2-) is diamagnetic while [Cr(NH(3))(6)]^(3+) is paramagne...
Text Solution
|
- [Cr(NH3)6]^(3+) is paramagnetic while [Ni(CN)4]^(2-) is diamagnetic. E...
Text Solution
|
- How many of the following are diamagnetic? [Ag(NH(3))(2)]^(+),[Cd(NH(3...
Text Solution
|
- [Cr(NH(3))(6)]^(3+) अनुचुम्बकीय है, जबकि [Ni(CN)(4)]^(2-) प्रतिचुम्बकी...
Text Solution
|
- [Ni(CN)(4)]^(2-) is diamagnetic while [Cr(NH(3))(6)]^(3+) is paramagne...
Text Solution
|
- [Cr(NH(3))(6)]^(3+) is paramagnetic while [Ni(CN)(4))]^(2-) is diamagn...
Text Solution
|
- [Cr(NH(3)(6)]^(3+) " अनुचुम्बकीय है , जबकि " [Ni(CN)(4)]^(2-) प्रतिच...
Text Solution
|
- [Cr(NH(3))(6)]^(3+) is paramagnetic while [Ni(CN)(4)]^(2-) is diamagne...
Text Solution
|
- Based VB theory explain why [Cr(NH3)6]^(3+) is paramagnetic , while [...
Text Solution
|