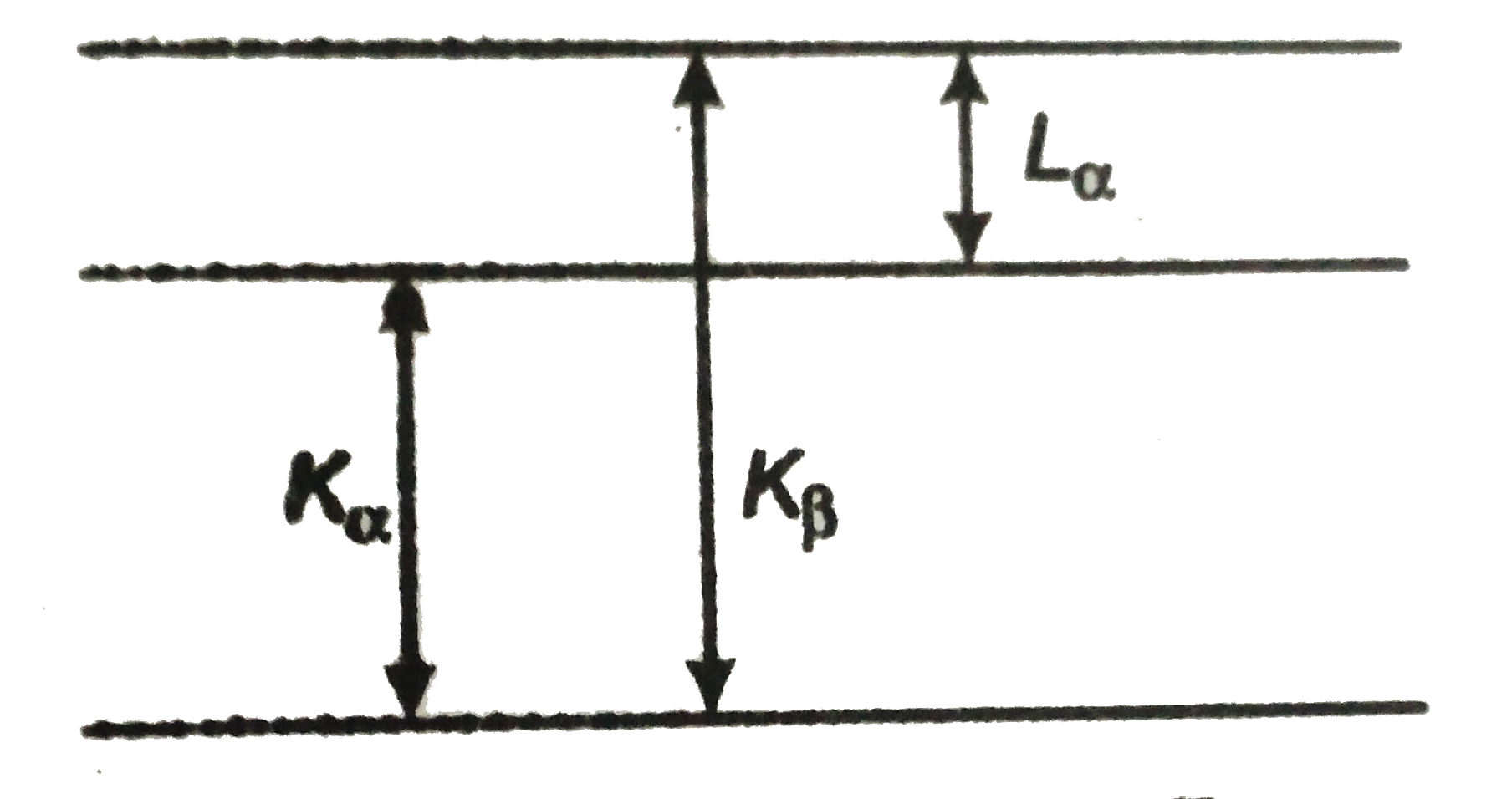
Text Solution
Verified by Experts
Topper's Solved these Questions
MODERN PHYSICS - 1
DC PANDEY|Exercise Level 2 Single Correct|22 VideosMODERN PHYSICS - 1
DC PANDEY|Exercise Level 2 More Than One Correct|6 VideosMODERN PHYSICS - 1
DC PANDEY|Exercise Level 1 Objective|37 VideosMODERN PHYSICS
DC PANDEY|Exercise Integer Type Questions|17 VideosMODERN PHYSICS - 2
DC PANDEY|Exercise Level 2 Subjective|10 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-MODERN PHYSICS - 1-Level 1 Subjective
- For a given element the wavelength of the Kalpha- line is 0.71 nm and ...
Text Solution
|
- The energy of the n=2 state in a given element is E2 =-2870 eV. Given ...
Text Solution
|
- 1.5 mW of 400 nm light is directed at a photoelectric cell. If 0.1% of...
Text Solution
|
- A photon has momentum of magnitude 8.24xx10^(-28) kg-m//s. (a) What is...
Text Solution
|
- A 75 W light source emits light of wavelength 600 nm. (a) Calculate th...
Text Solution
|
- An excited nucleus emits a gamma-ray photon with energy of 2.45 MeV. ...
Text Solution
|
- (a) A proton is moving at a speed much less than the speed of light. I...
Text Solution
|
- A parellel beam of monochromatic light of wavelength 500 nm is incide...
Text Solution
|
- A beam of white light is incident normaly on a plane surface absorbin...
Text Solution
|
- A parallel beam of monochromatic light of wavelength 663 nm is inciden...
Text Solution
|
- wavelength of Bullet. Calculate the de-Brogli wavelength of a 5.00 g b...
Text Solution
|
- (a) An electron moves with a speed of 4.70xx10^6 m//s. What is its de...
Text Solution
|
- An electron has a de Broglie wavelength of 2.80xx10^(-10) m. Determine...
Text Solution
|
- Find de-Broglie wavelength corresponding to the root-mean square velo...
Text Solution
|
- An electron, in a hydrogen like atom , is in excited state. It has a t...
Text Solution
|
- In the Bohr model of the hydrogen atom, what is the de-Broglie wavelen...
Text Solution
|
- The binding energy of an electron in the gorund state of He atom is ...
Text Solution
|
- Hydrogen atom is its ground state is excited by means of monochromatic...
Text Solution
|
- A doubly ionized lithium atom is hydrogen like with atomic number 3. F...
Text Solution
|
- Find the quantum number n corresponding to nth excited state of He^(++...
Text Solution
|