A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOLID STATE-Ex 1.2 (Objective)
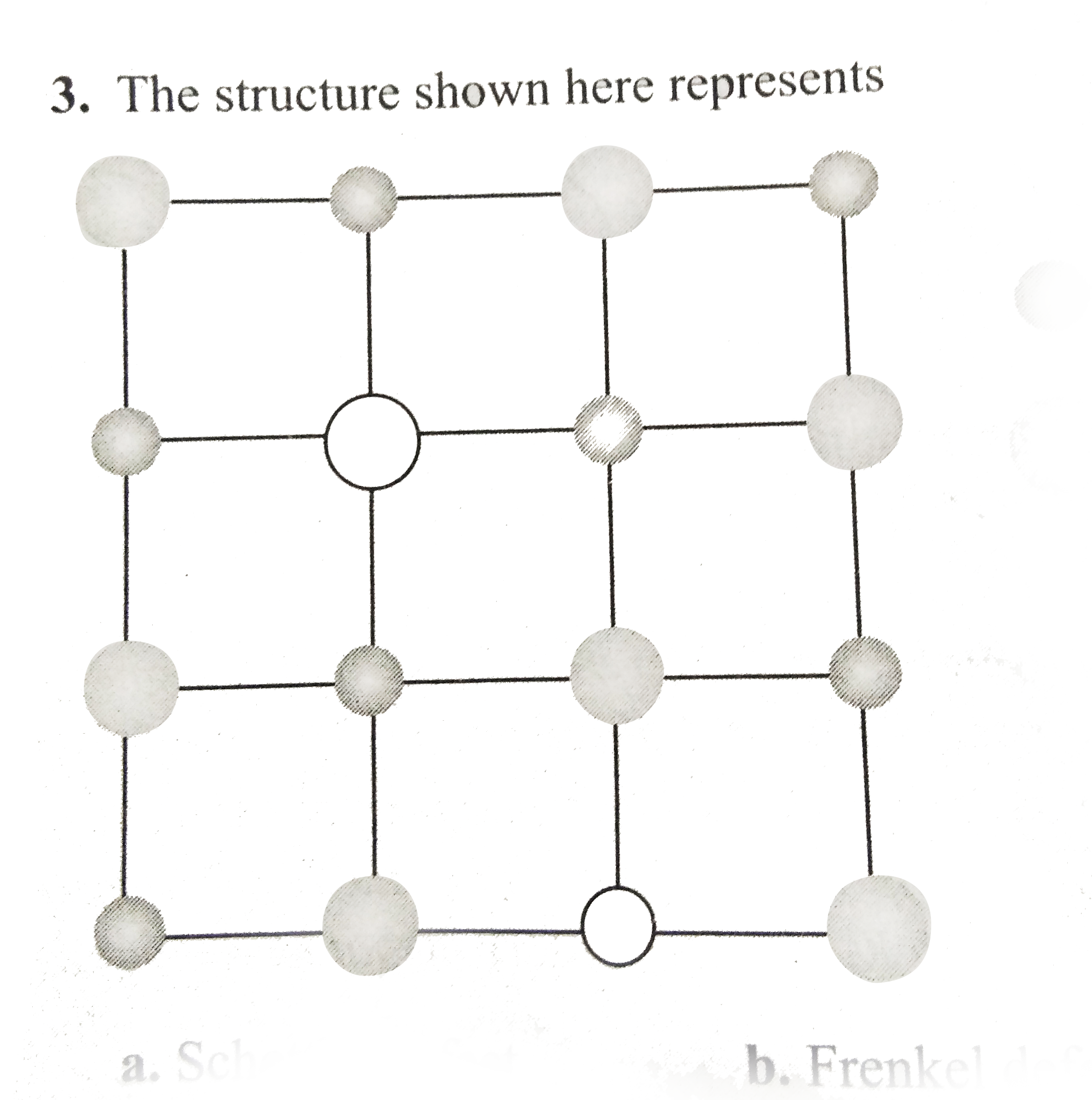
- The structure shown here represents
Text Solution
|
- The structure shwon here represents
Text Solution
|
- In AgCl, the Ag^(o+) ions are deisplaced from their lattice position t...
Text Solution
|
- NaCl shows Schottky defects and AgCl shows Frekel defects. Their elect...
Text Solution
|
- Amorphous solids are classified as
Text Solution
|
- Due of Frenkel defect
Text Solution
|
- Which of the following statement is//are correct?
Text Solution
|
- Which of the following statement is//are correct?
Text Solution
|
- Which of the following statement is//are correct?
Text Solution
|