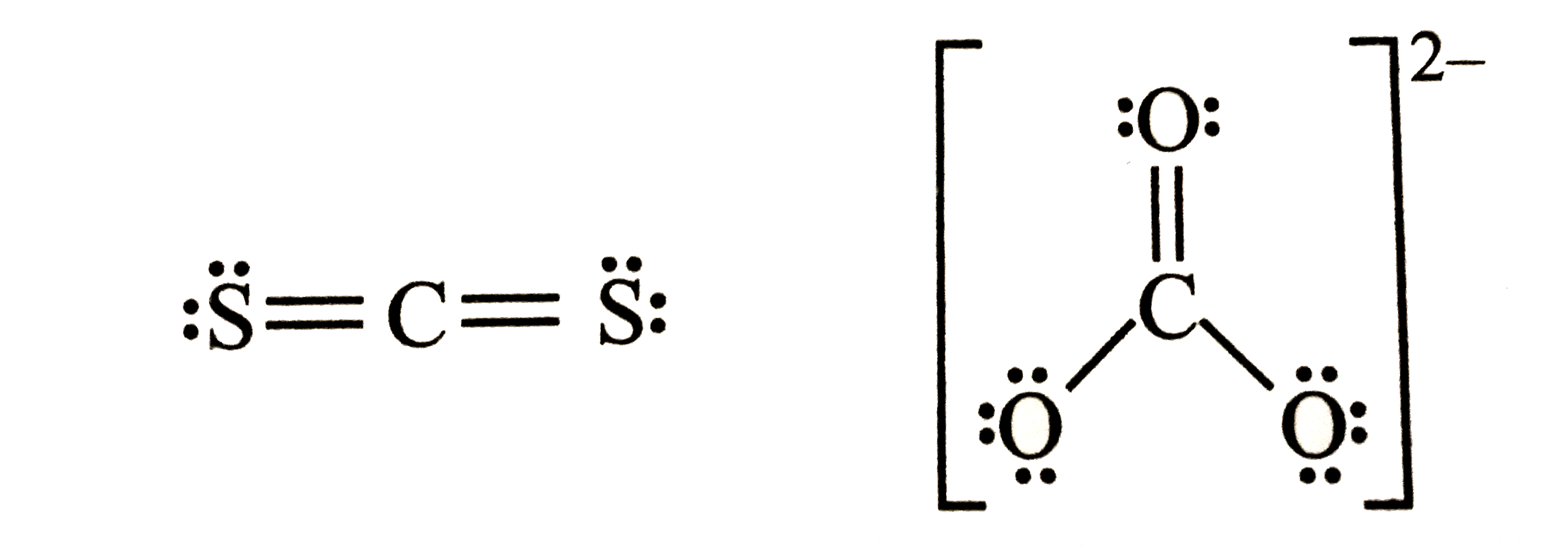
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Vsepr Theory|15 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Valence Bond Theory|7 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Bond Parameters|13 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-CHEMICAL BONDING & MOLECULAR STRUCTURE-MCQs
- What is the formal charge on carbon atom in the following two structur...
Text Solution
|
- The given structures I, II and III of carbonate ion represent
Text Solution
|
- Although F is more electronegative than H, the resultant dipole moment...
Text Solution
|
- Which of the following is the most stable state when two atoms come cl...
Text Solution
|