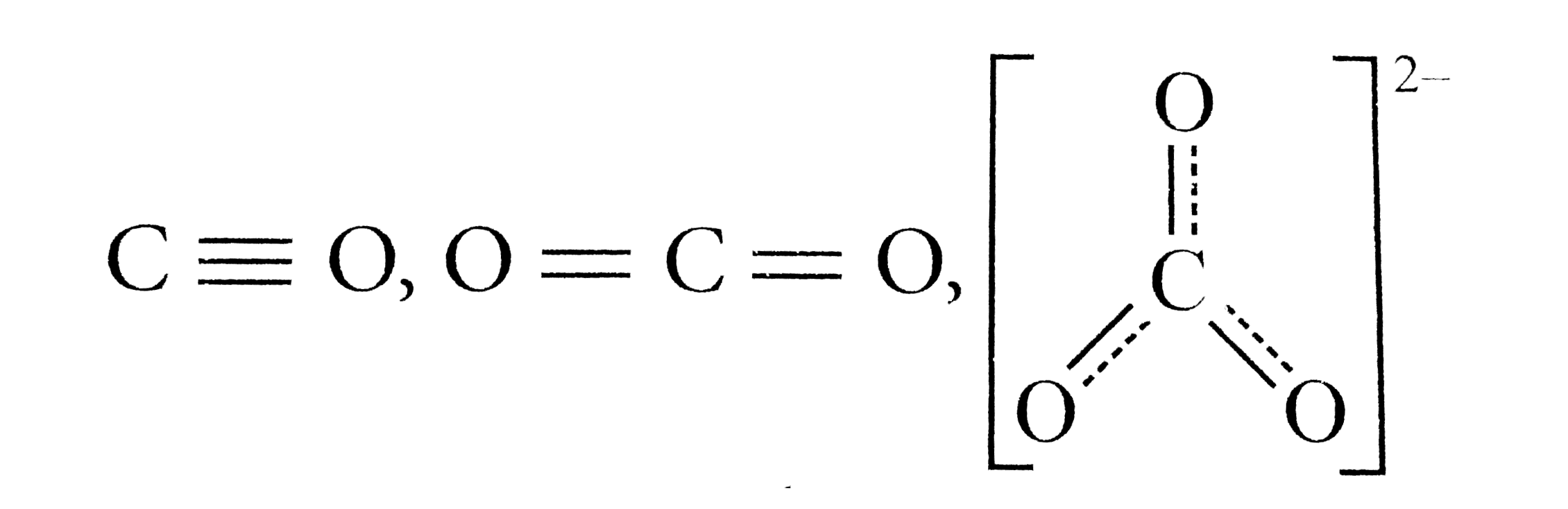A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise MCQs|4 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Vsepr Theory|15 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS|Exercise Ionic And Electrovalent Bond|4 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-CHEMICAL BONDING & MOLECULAR STRUCTURE-Bond Parameters
- The correct order of decreasing C-O bond length of (i) CO,(II)CO(3)^(2...
Text Solution
|
- The C -C bond length is 1.54 Å C =C bond length is 1.33 Å What is the ...
Text Solution
|
- Which has the strongest bond?
Text Solution
|
- Match the bond enthalpies given in column II with the molecules given ...
Text Solution
|
- Which of the following molecules does not show any resonating structur...
Text Solution
|
- The canonical or resonating structures of a molecule required to descr...
Text Solution
|
- Arrange the following in decreasing order of dipole moment (a) Tolue...
Text Solution
|
- Which of the following is non polar species.
Text Solution
|
- Diatomic molecule has a dipole moment of 1.2D If its bond 1.0 Å what f...
Text Solution
|
- Which of the following are arranged in the decreasing order of dipole ...
Text Solution
|
- The dipoles moment of NF(3) is less than NH(3) because
Text Solution
|
- In water molecule, the two O - H bonds are oriented at an angle of 104...
Text Solution
|
- Give the decreasing pH of aqueous solution of the following compounds ...
Text Solution
|