Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP 15 ELEMENTS (VERY SHORT ANSWER QUESTIONS)|60 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP - 16 ELEMENTS (VERY SHORT ANSWER QUESTIONS)|37 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 VideosPOLYMERS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|6 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-P-BLOCK ELEMENTS -INTEXT QUESTIONS
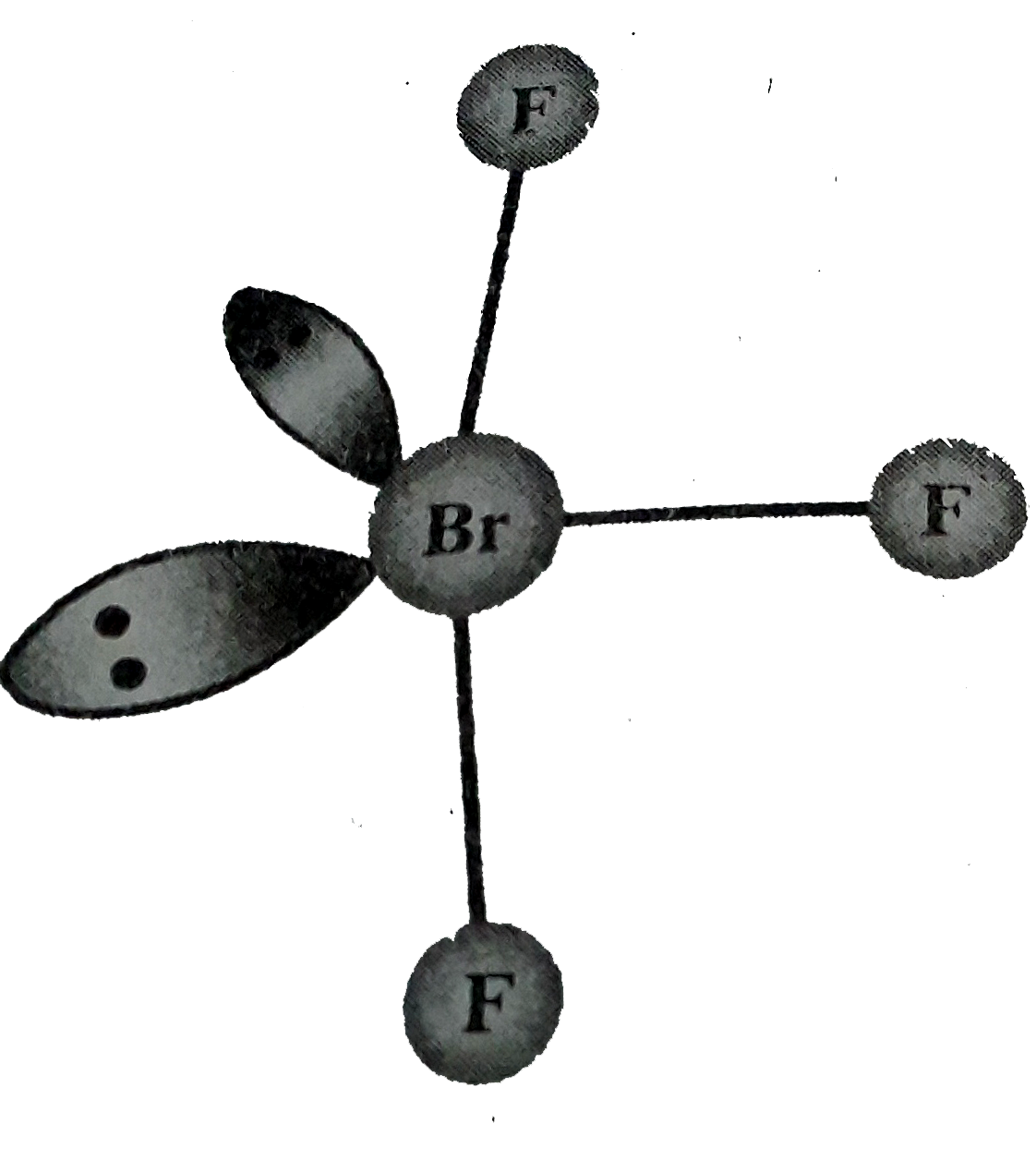
- Discuss the molecular shape of "BrF"(3) on the basis of VSEPR theory.
Text Solution
|
- Why are pentahalides more covalent than trihalides ?
Text Solution
|
- Why is "BiH"(3) the strongest reducing agent amongst all the hydrides ...
Text Solution
|
- Why is N(2) less reactive at room temperature ?
Text Solution
|
- Mention the conditions required to maximise the yield of ammonia.
Text Solution
|
- How does ammonia react with a solution of "Cu"^(2+) ?
Text Solution
|
- What is the covalence of nitrogen in "N"(2)"O"(5) ?
Text Solution
|
- Bond angle in "PH"(4)^(" "+) is higher than that in "PH"(3). Why ?
Text Solution
|
- What happens when white phosphorus is heated with concentrated NaOH s...
Text Solution
|
- What happens when "PCl"(5) is heated ?
Text Solution
|
- Write a balanced equation for the hydrolytic reaction of PCl(5) in hea...
Text Solution
|
- List the important sources of sulphur.
Text Solution
|
- Write the order of thermal stability of the hydrides of group 16 eleme...
Text Solution
|
- Why is H(2)O a liquid and H(2)S a gas ?
Text Solution
|
- Which of the following does not react with oxygen directly ? Zn, Ti, P...
Text Solution
|
- Complete the following reactions. i) C(2)H(4)+O(2)to ii) 4" Al"+3O(2...
Text Solution
|
- Why does O(3) act as a powerful oxidising agent ?
Text Solution
|
- How is O(3) estimated quantitatively ?
Text Solution
|
- What happens when sulphur dioxide is passed through an aqueous solutio...
Text Solution
|
- Comment on the nature of two S - O bonds formed is SO(2) molecule. Are...
Text Solution
|
- How is the presence of SO(2) detected ?
Text Solution
|