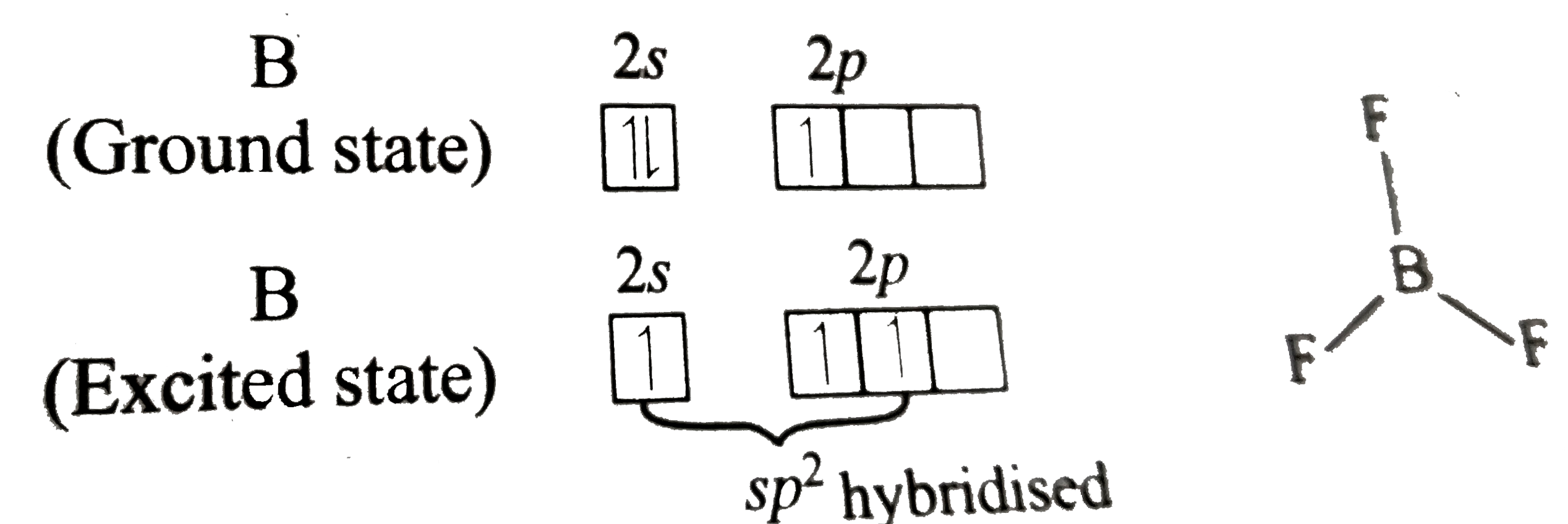
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise (Single Correct)|86 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise (Assertion-Reasoning)|13 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise (Linked Comprehension)|37 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive|6 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 13 - BORON FAMILY-Exercise (Multiple Correct)
- Which of the following metals are extracted by using Al as reducing ag...
Text Solution
|
- Aluminium becomes passive in :
Text Solution
|
- Which of the following statements are true for H(3)BO(3) ?
Text Solution
|
- In the following reaction. 2X + B(2)H(6) rarr [BH(2)(X)(2)]^(oplus) ...
Text Solution
|
- Possible oxidation states of boron family elements are :
Text Solution
|
- Select the correct statements about diborane :
Text Solution
|
- Orthoboric acid (H(3)BO(3)) and metaboric acid (HBO(2)) differ in resp...
Text Solution
|
- Which of the following element do not form carbide ?
Text Solution
|
- Diborane reacts with ammonia under different conditions to give :
Text Solution
|
- Why the atomic radius of gallium is less than that of Al?
Text Solution
|
- BF(3) is.
Text Solution
|
- Which of the following compounds (s) undergo disproportionation in aqu...
Text Solution
|
- Which of the following statements are correct for Al ?
Text Solution
|
- Boric acid is prepared from borax by the action of.
Text Solution
|
- Alumina is.
Text Solution
|
- Which of the following are incorrect statements ?
Text Solution
|
- Potash alum is used as a
Text Solution
|
- Which of the following minerals contain aluminium ?
Text Solution
|
- Boranes have general formula :
Text Solution
|
- Double sulphates of a divalent and trivalent metal crystallised with 2...
Text Solution
|