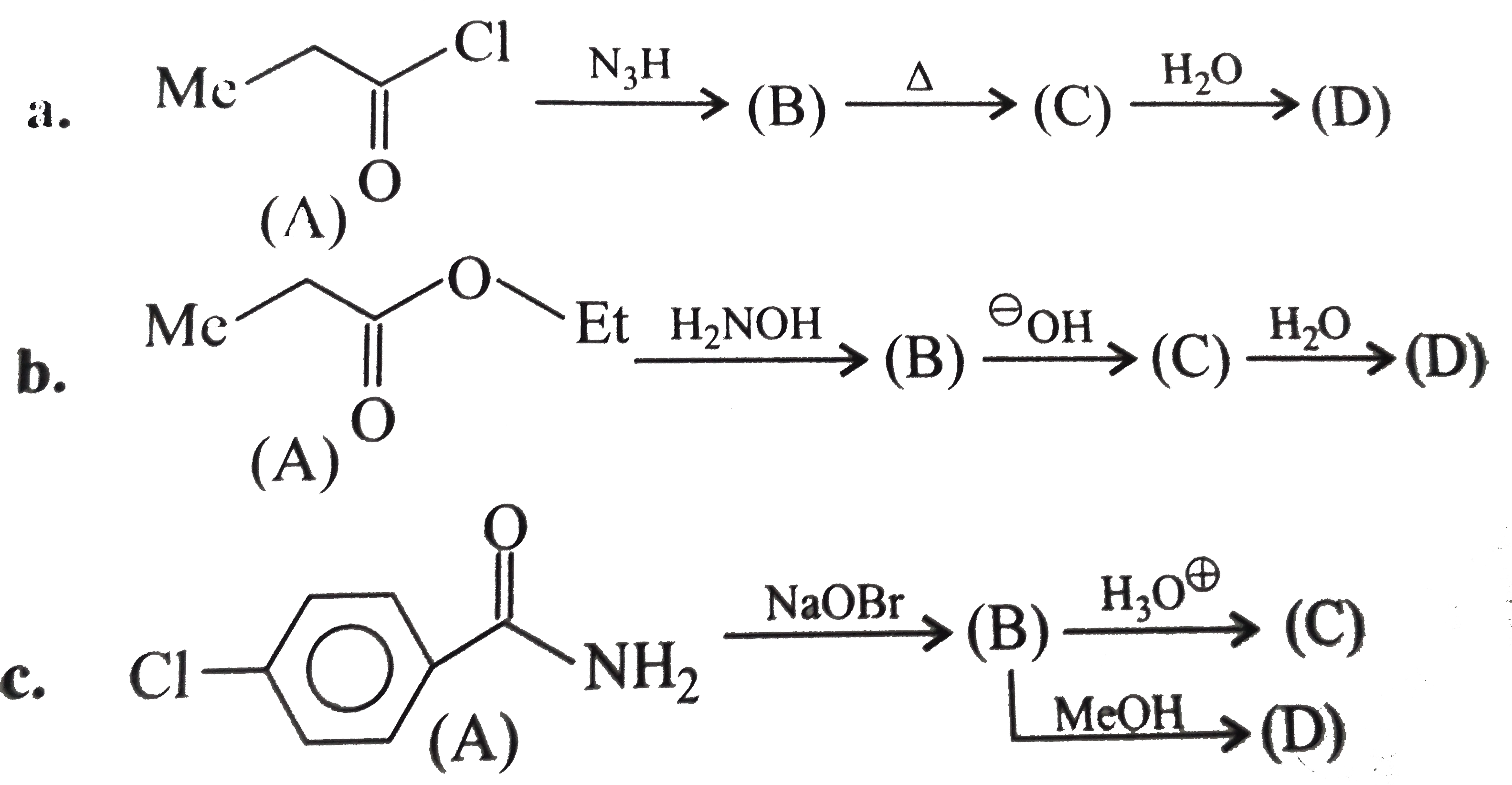
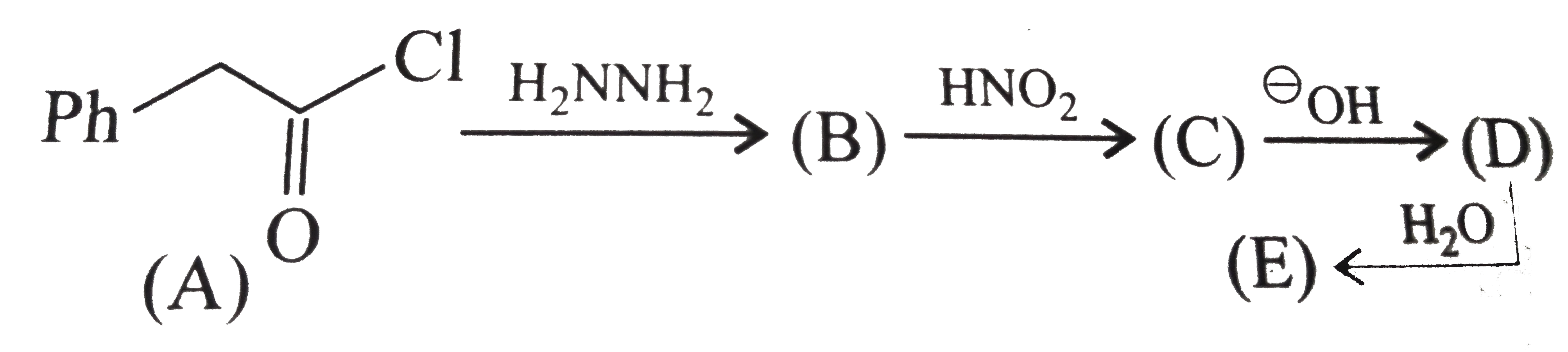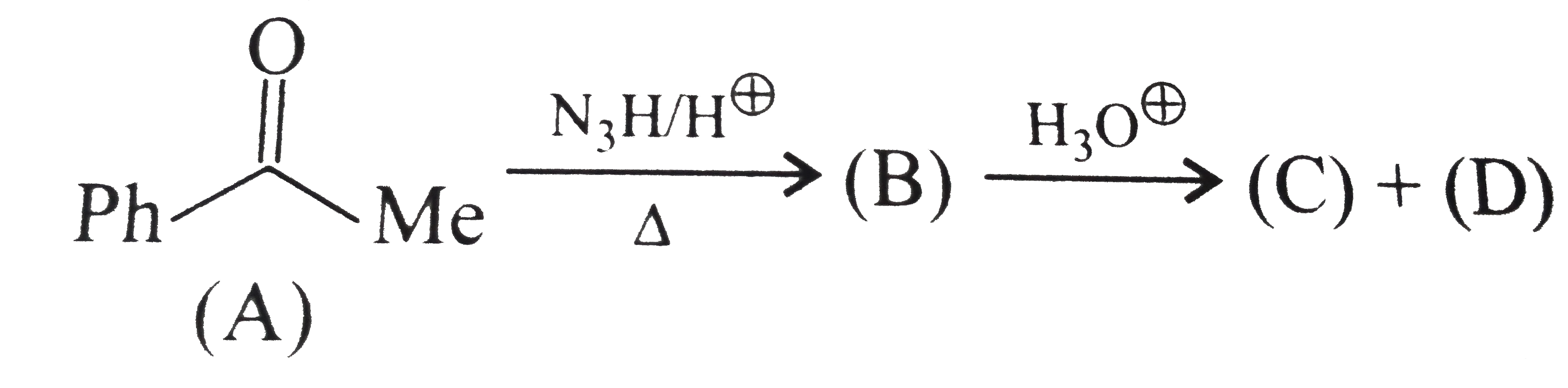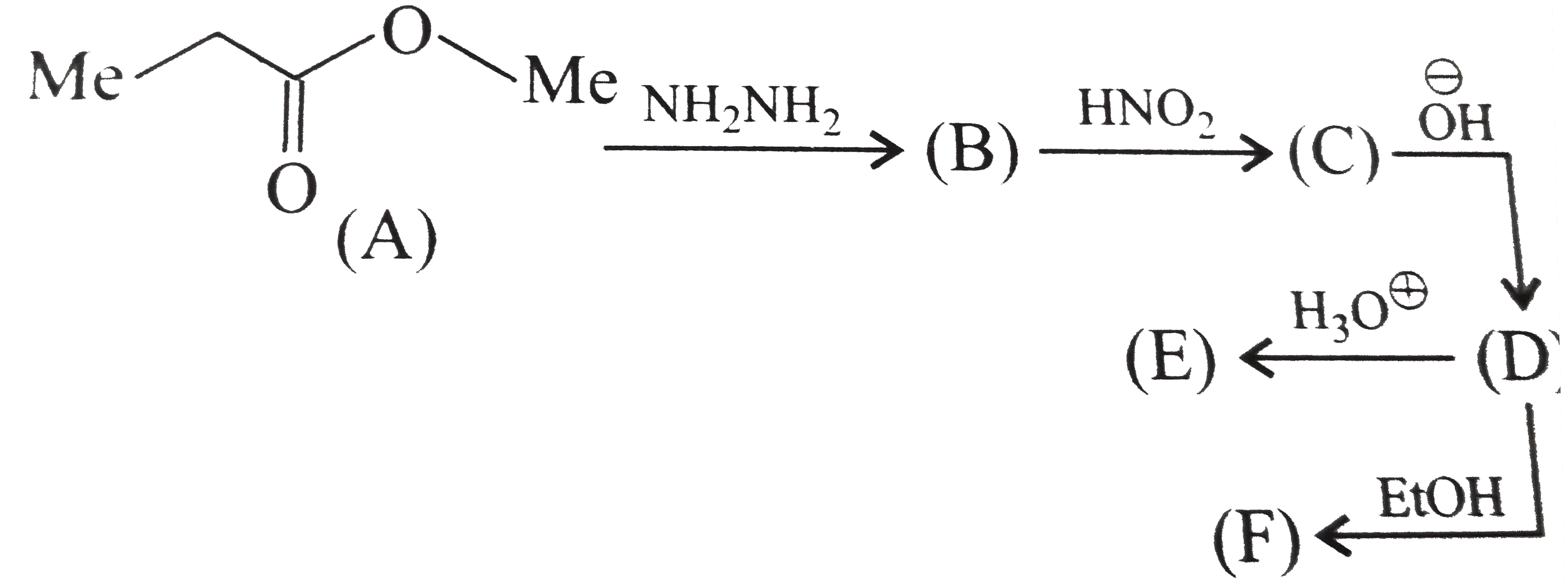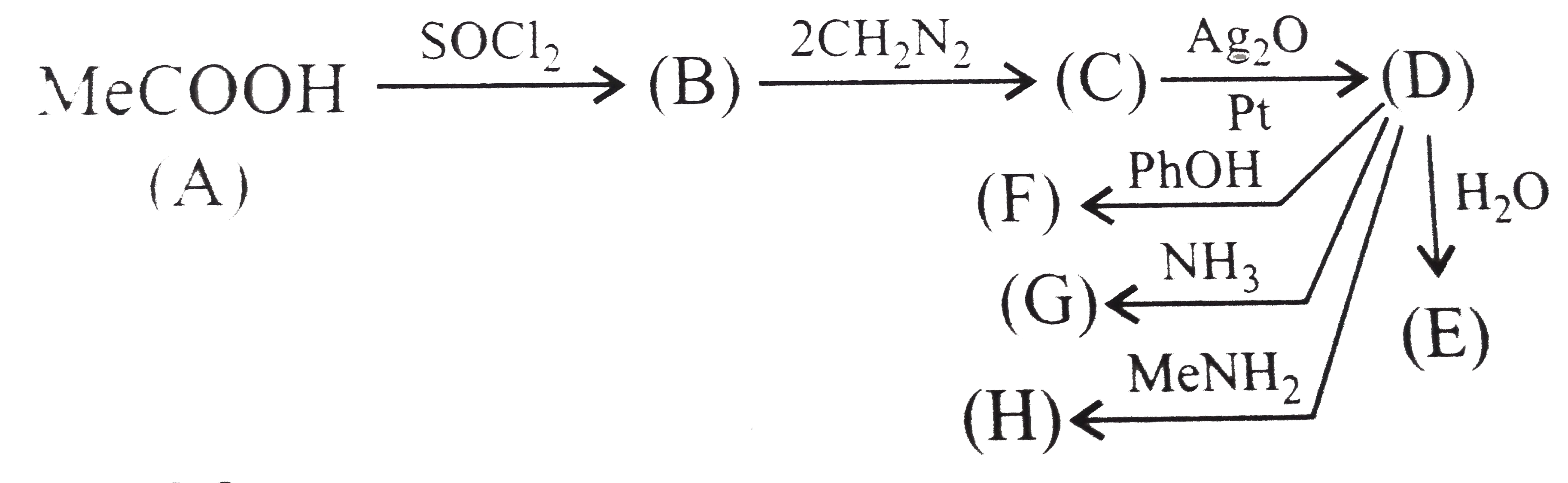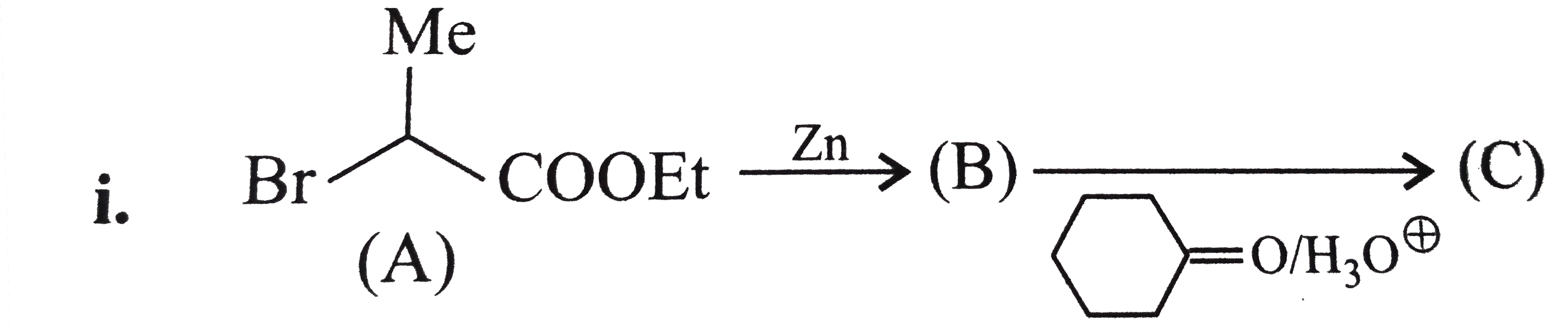Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Ex 8.2|79 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Viva Voce Questions And Part-A (Analysis Of Anions)|30 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|34 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Ex 5.1 (Objective)|14 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise SUBJECTIVE TYPE|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-QUALITATIVE INORGANIC SALT ANALYSIS-Ex 8.1
- Identify (A) to (H) Mineral (A) overset(dil.H(2)SO(4))rarr(B) +(C) +...
Text Solution
|
- An aqueous of salt (A) gives a white crystalline precipitate (B) wit...
Text Solution
|
- A white amorphous powder (A) when heated given by a colourlesss gas...
Text Solution
|
- Compound (A) is a light green crystalline solid it gives the followi...
Text Solution
|
- Identify A to E
Text Solution
|
- Identify A to F
Text Solution
|
- Identify A to D
Text Solution
|
- Identify A to L
Text Solution
|
- Identify A to G
Text Solution
|
- Identify A to D
Text Solution
|
- Identify A to E
Text Solution
|
- Identify A to H
Text Solution
|
- Identify A to C
Text Solution
|
- Identify A to D
Text Solution
|