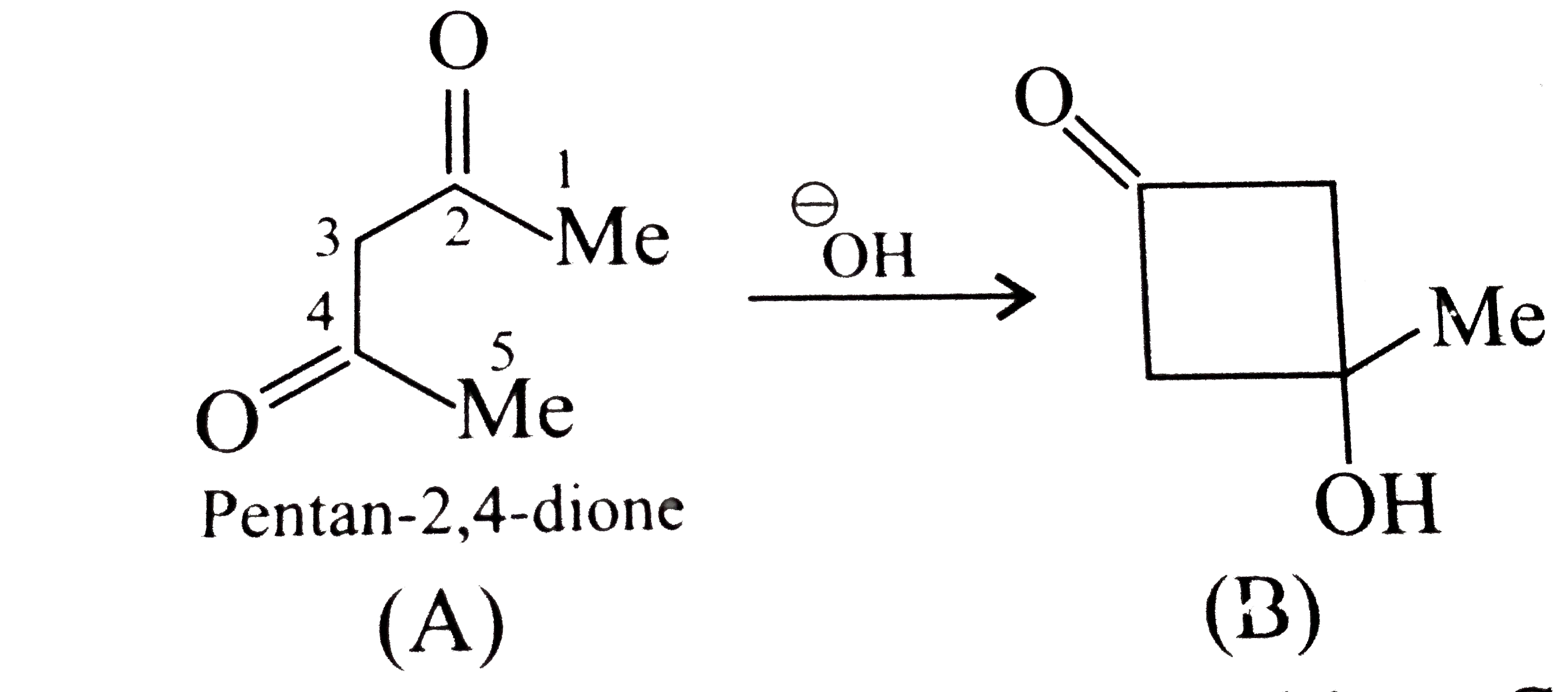A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY|Exercise Archives Objective|10 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY|Exercise Archives Multiple Correct|8 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY|Exercise Exercises Single Correct|72 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY|Exercise Archives Analytical And Descriptive|15 VideosAMINES
CENGAGE CHEMISTRY|Exercise QUESTION BANK|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES-Exercises Assertion-Reasoning
- Assertion (A): The following intramolecular aldol reaction occurs. ...
Text Solution
|
- Assertion (A): The equilibrium constant for cyanohydrin formation is =...
Text Solution
|
- Assertion (A): The following reaction occurs. Reason (R ): Underg...
Text Solution
|
- Assertion (A): Pentan-2-one on reaction with NaHSO(3) gives sodium bis...
Text Solution
|
- Assertion (A): The following reaction occurs. Reason (R ): The se...
Text Solution
|
- Assertion (A): The following cross Cannizzaro reaction occurs. Re...
Text Solution
|
- Assertion (A): The following intramolecular aldol reaction occurs. ...
Text Solution
|
- Assertion (A): The following reaction occurs. Reason (R ): The pr...
Text Solution
|
- Assertion (A): The reaction of 1 mol each of (MeCH==O) with glycerol ...
Text Solution
|
- Assertion (A): The reaction MeCOCI with MeCuLi or Me(2)Cd gives actone...
Text Solution
|