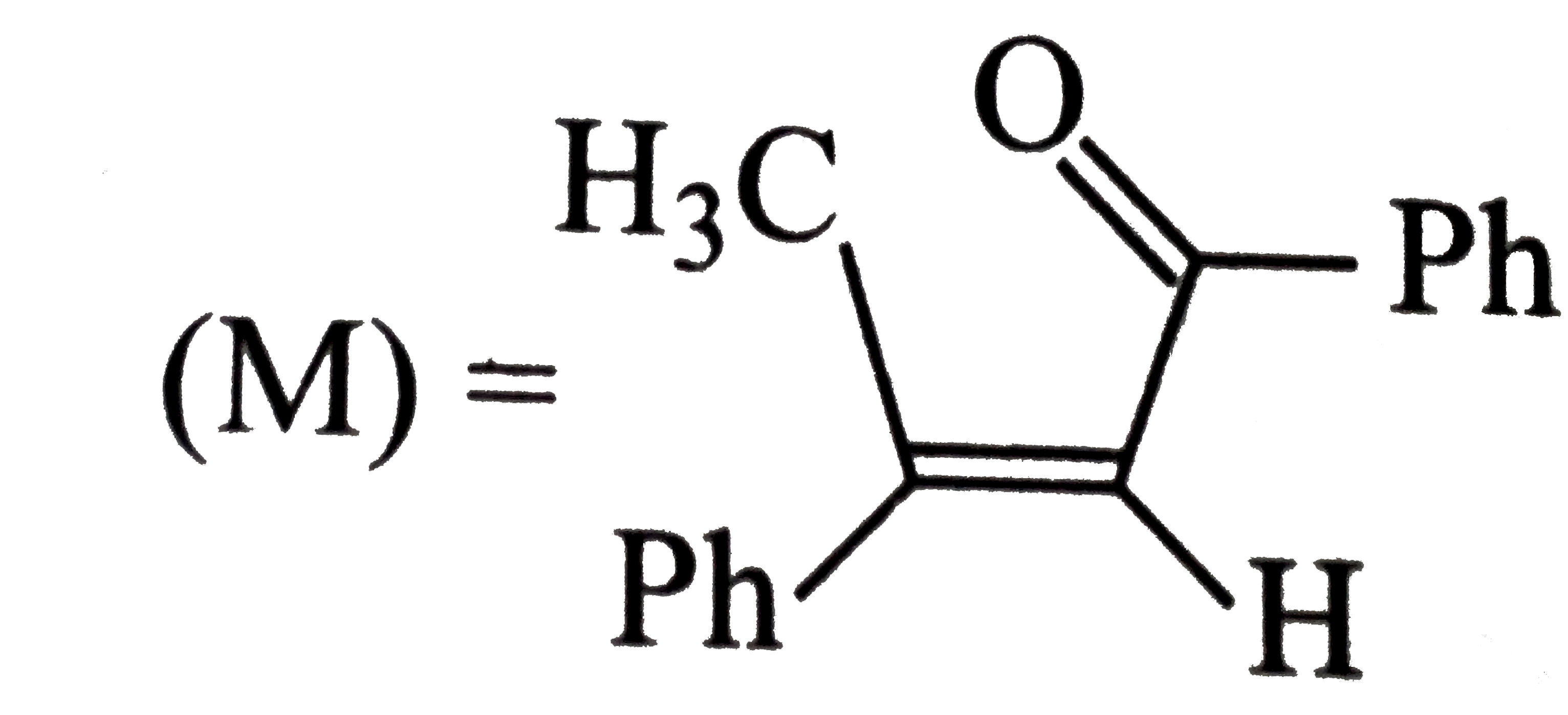
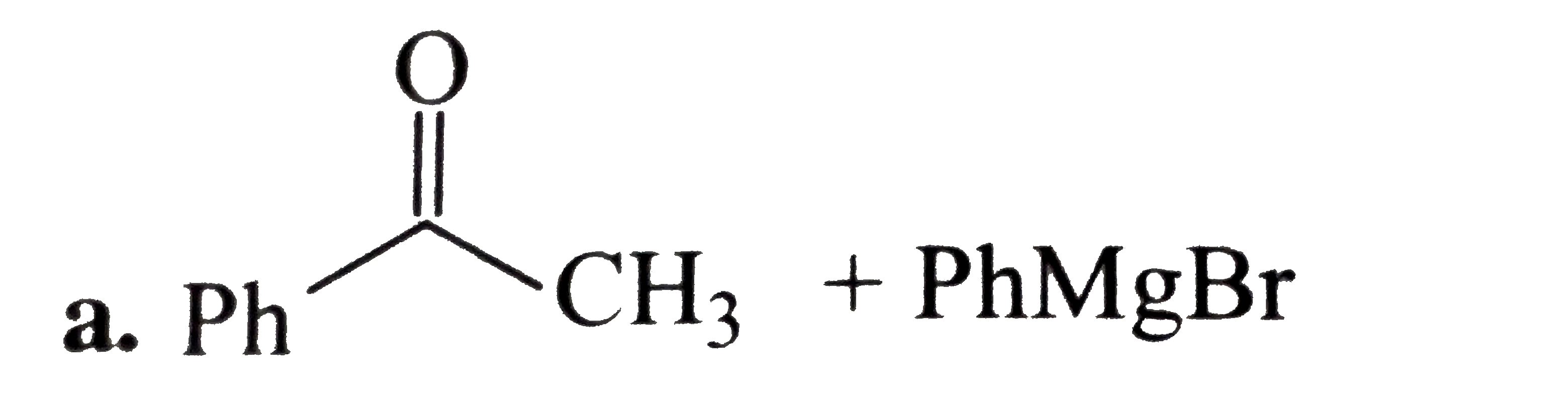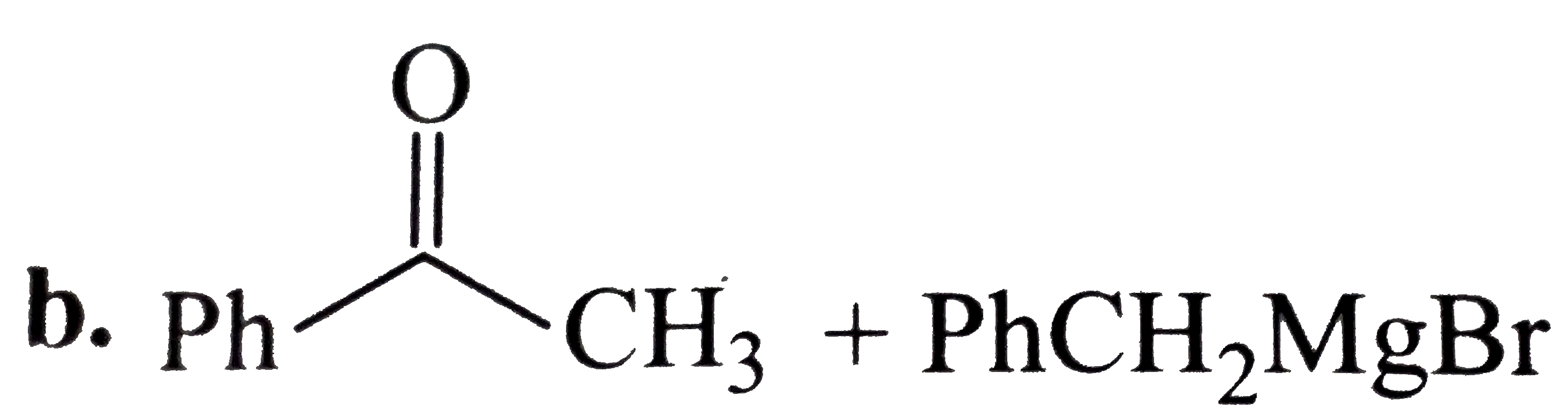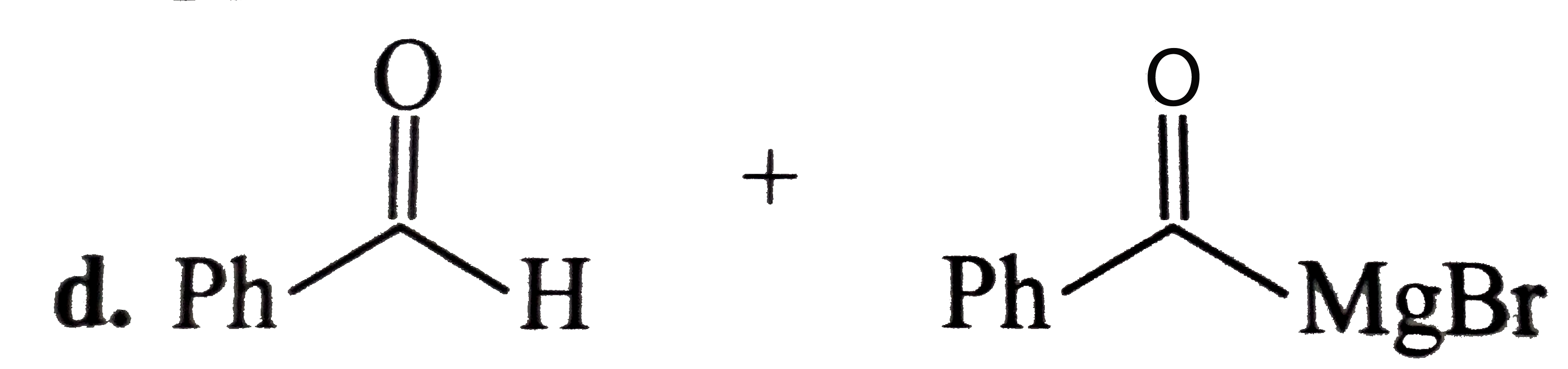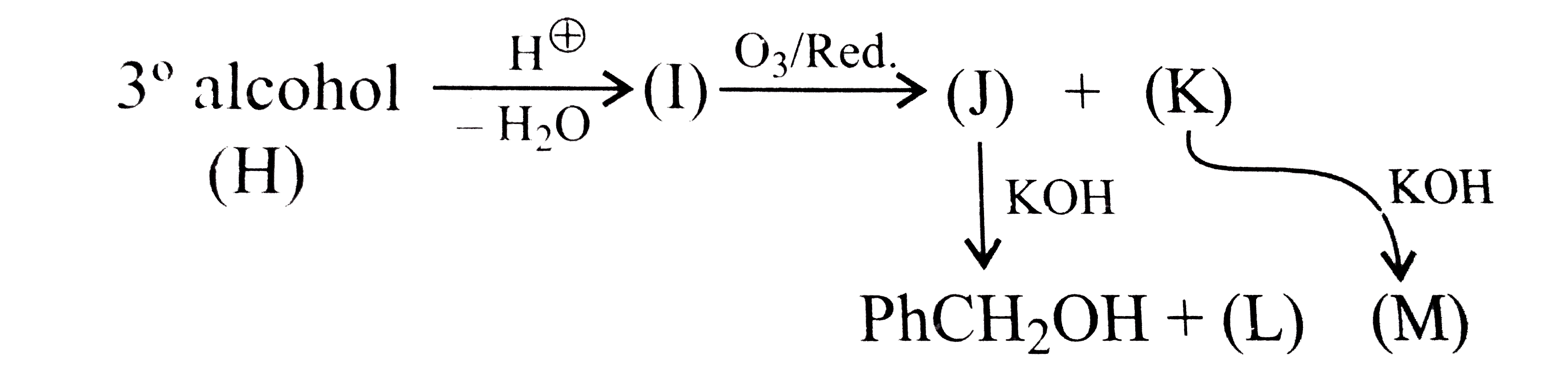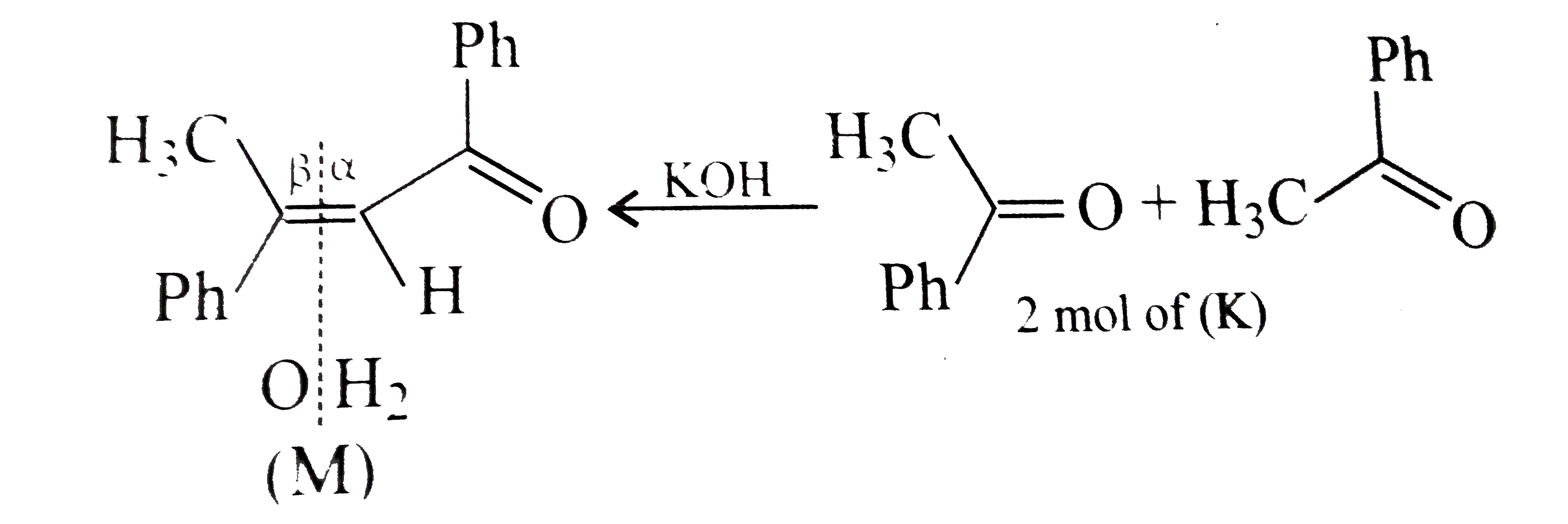A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY|Exercise Archives True And False|1 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY|Exercise Archives Analytical And Descriptive|15 VideosAMINES
CENGAGE CHEMISTRY|Exercise QUESTION BANK|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES-Archives Linked Comprehension
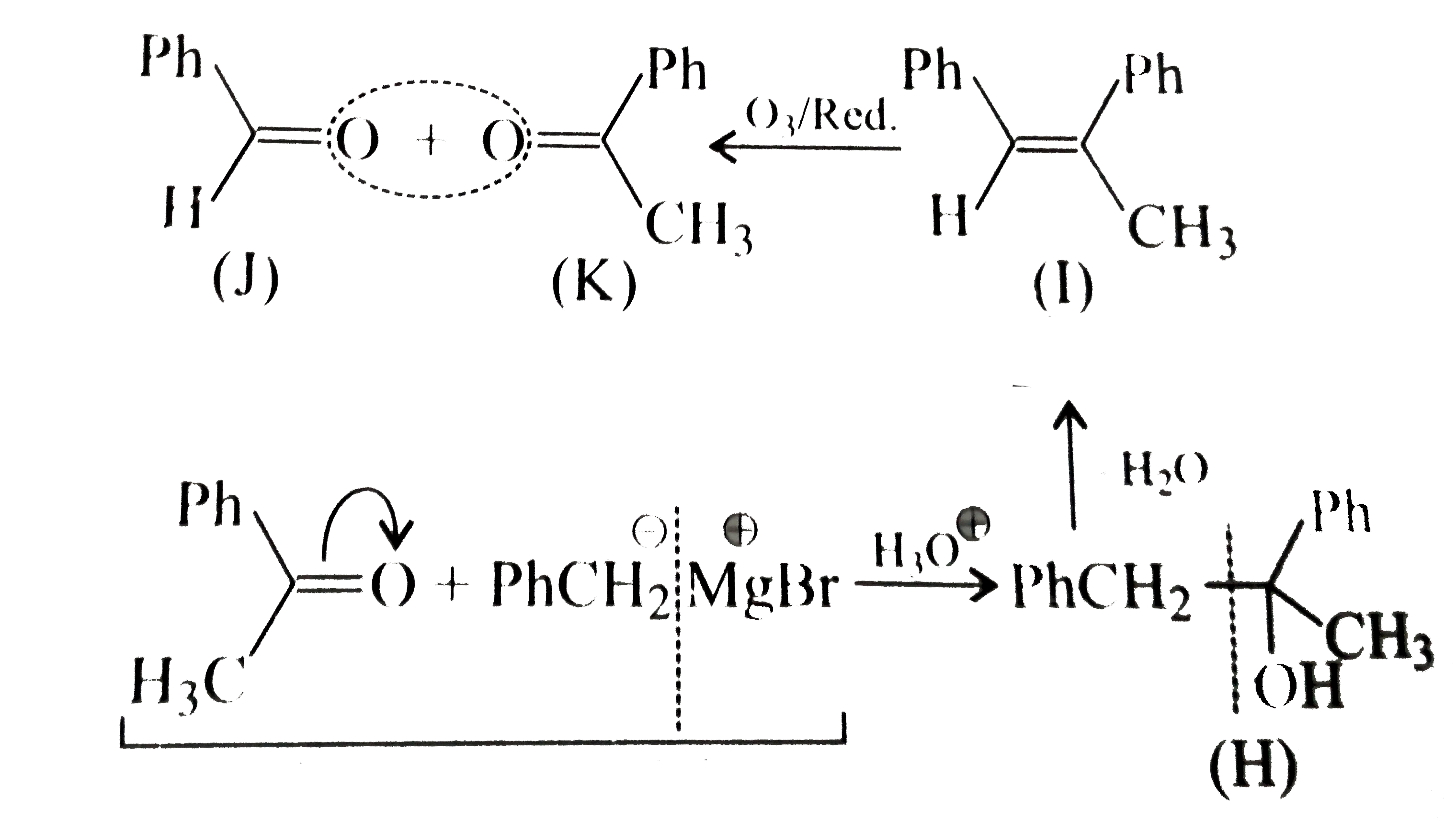
- A tertiary alcohol (H) upon acid-catalysed dehydration gives a product...
Text Solution
|
- A tertiary alcohol (H) upon acid-catalysed dehydration gives a product...
Text Solution
|
- A tertiary alcohol (H) upon acid-catalysed dehydration gives a product...
Text Solution
|
- A carbonyl compound (P) which gives positive iodoform test, undergoes ...
Text Solution
|
- A carbonyl compound (P) which gives positive iodoform test, undergoes ...
Text Solution
|
- A carbonyl compound (P) which gives positive iodoform test, undergoes ...
Text Solution
|