A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises (Assertion-Reasoning)|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Single Correct)|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|35 VideosBIOMOLECULES, POLYMERS AND CHEMISTRY IN EVERYDAY LIFE
CENGAGE CHEMISTRY|Exercise QUESTION BANK|8 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Archives Subjective|23 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Exercises (Singlecorrect)
- Which of the following tests would help in the distinction of HCOOH an...
Text Solution
|
- Which of the following does not contain (-COOH) group ?
Text Solution
|
- Acids do not give the characteristic reaction of (C=O) group because o...
Text Solution
|
- The addition of Hbr to CH2 = CHCOOH results in the formation of.
Text Solution
|
- Mark the correct order of increasing reactivity.
Text Solution
|
- CH3COOH overset(Br2//P) rarr (Y) overset((i) KCN) underset((ii) H3 O^...
Text Solution
|
- Westrosol overset(90 %H2 SO4)rarr(X) overset(NH3)rarr (Y). Here, Y is ...
Text Solution
|
- Electrolytic reduction of oxalic acid produces :
Text Solution
|
- When glycolic acid is subjected to reduction with HI, the product form...
Text Solution
|
- When lactic acid is subjected to oxidation with Fenton's reagent, the ...
Text Solution
|
- Which of the following reagents is used for the preparation of citric ...
Text Solution
|
- Treatment of tartaric acid with Fenton's reagent gives :
Text Solution
|
- A hydrocarbon C6 H12 decolourises bromine solution and yields n-hexane...
Text Solution
|
- An organic liquid of the composition C4 H8 O2 yields a sodium salt of ...
Text Solution
|
- On heating calcium acetate and calcium formate, the product formed is ...
Text Solution
|
- CH3 CH=CHCHO is oxidised to CH3 CH=CHCOOH using :
Text Solution
|
- One mole of an organic compound is found to require only 0.5 mol of ox...
Text Solution
|
- Identify Z in the following series CH(2)=CH(2) overset(HBr)rarr X ov...
Text Solution
|
- Which one of the following has the maximum acid strength ?
Text Solution
|
- Formic acid is obtained when :
Text Solution
|
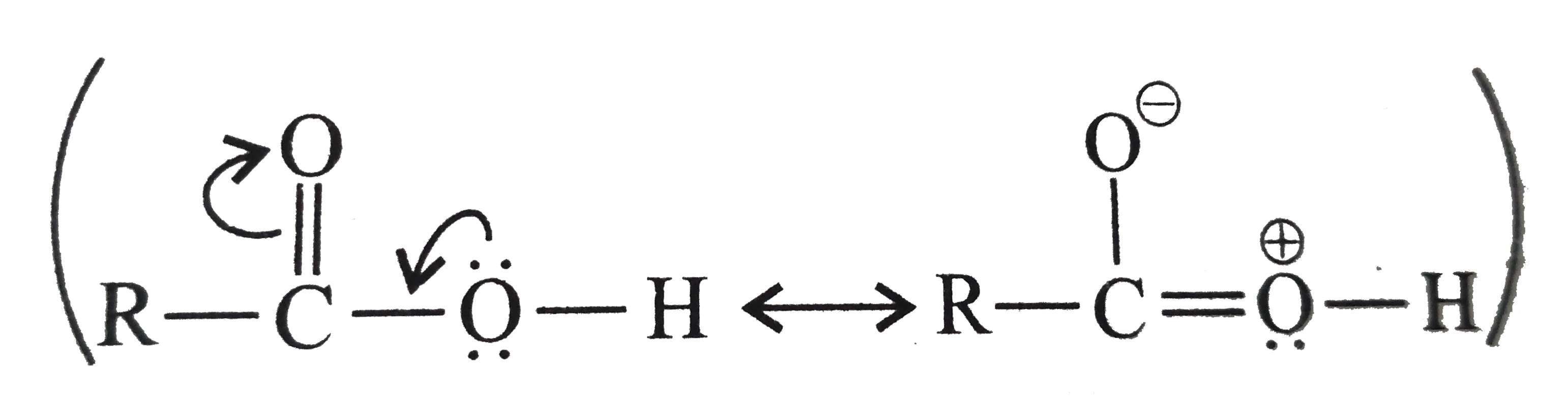 .
.