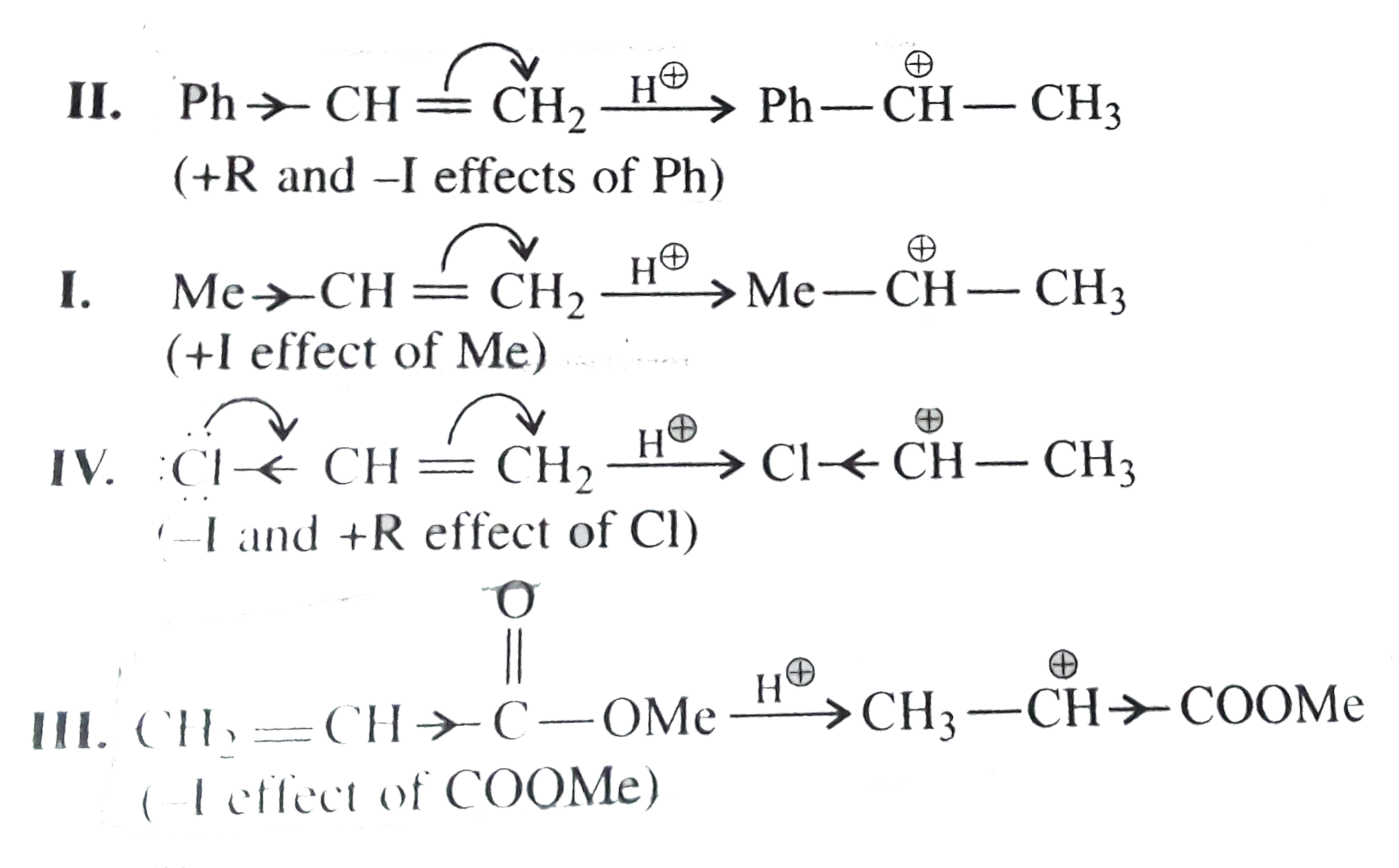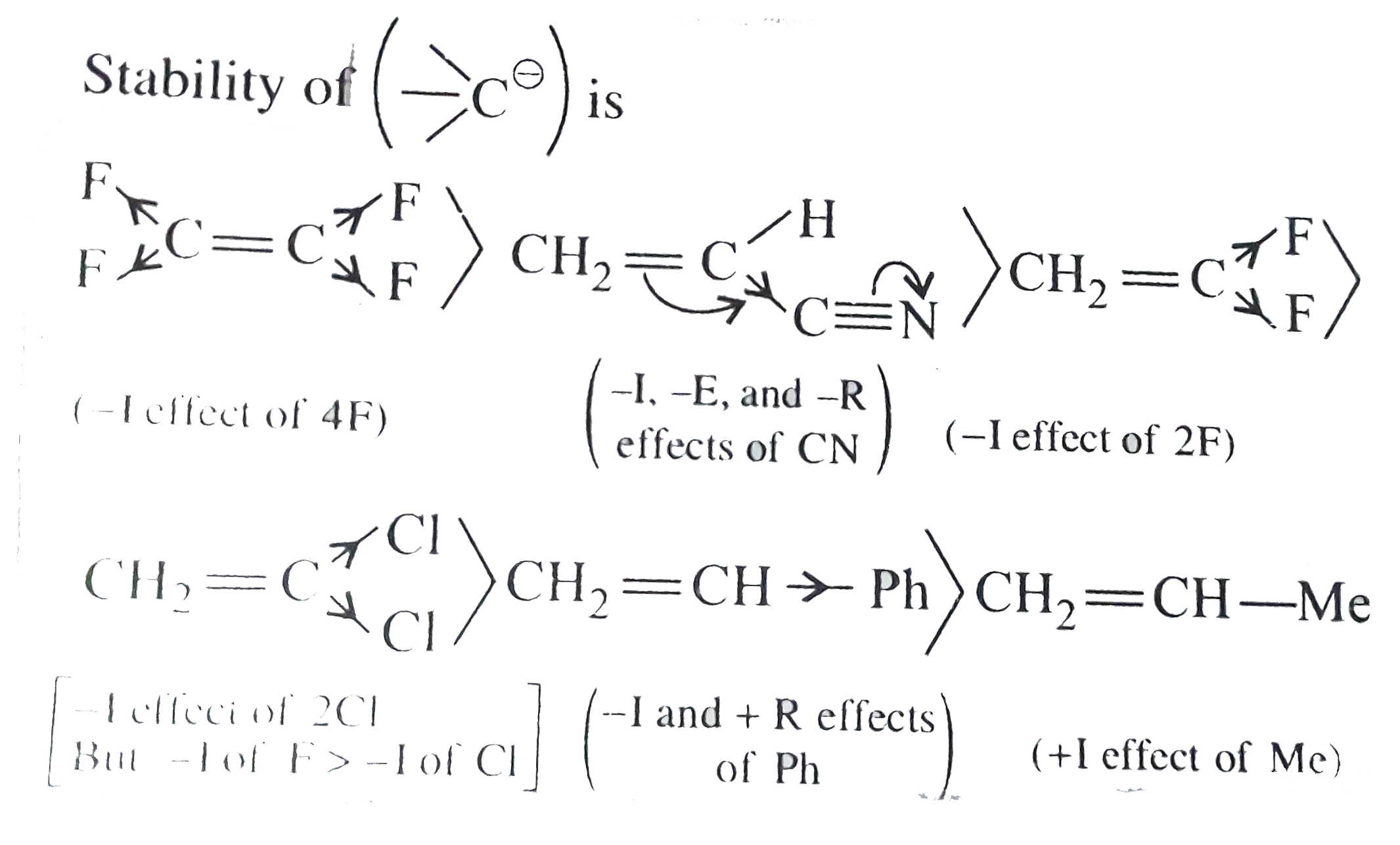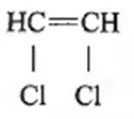Text Solution
Verified by Experts
|
Topper's Solved these Questions
SYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY|Exercise Exercises Concept Application|20 VideosView PlaylistSYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY|Exercise Exercises Linked Comprehension|30 VideosView PlaylistSYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY|Exercise Exercises Assertion Reasoning|10 VideosView PlaylistSURFACE CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|2 VideosView Playlist
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SYNTHETIC AND NATURAL POLYMERS-Solved Example
- (a)Give the decreasing order of reactivites of the following monmers t...
Text Solution
|
Playing Now - Explain why propone polymerises in isotactic,syndiotactic and atactic ...
02:50
|
Play - (a) Explain the free radical polymerisation of styrene. (b) The foll...
03:48
|
Play - Explain why polymerisation of accrylonitrile is preferred under ationi...
Text Solution
|
Play - (a)Why is the purest monomer used in free radical polymerisation? (b...
Text Solution
|
Play - A copolymer of ethene and vinyl chloride contains alternate monomers f...
03:52
|
Play
 is
is