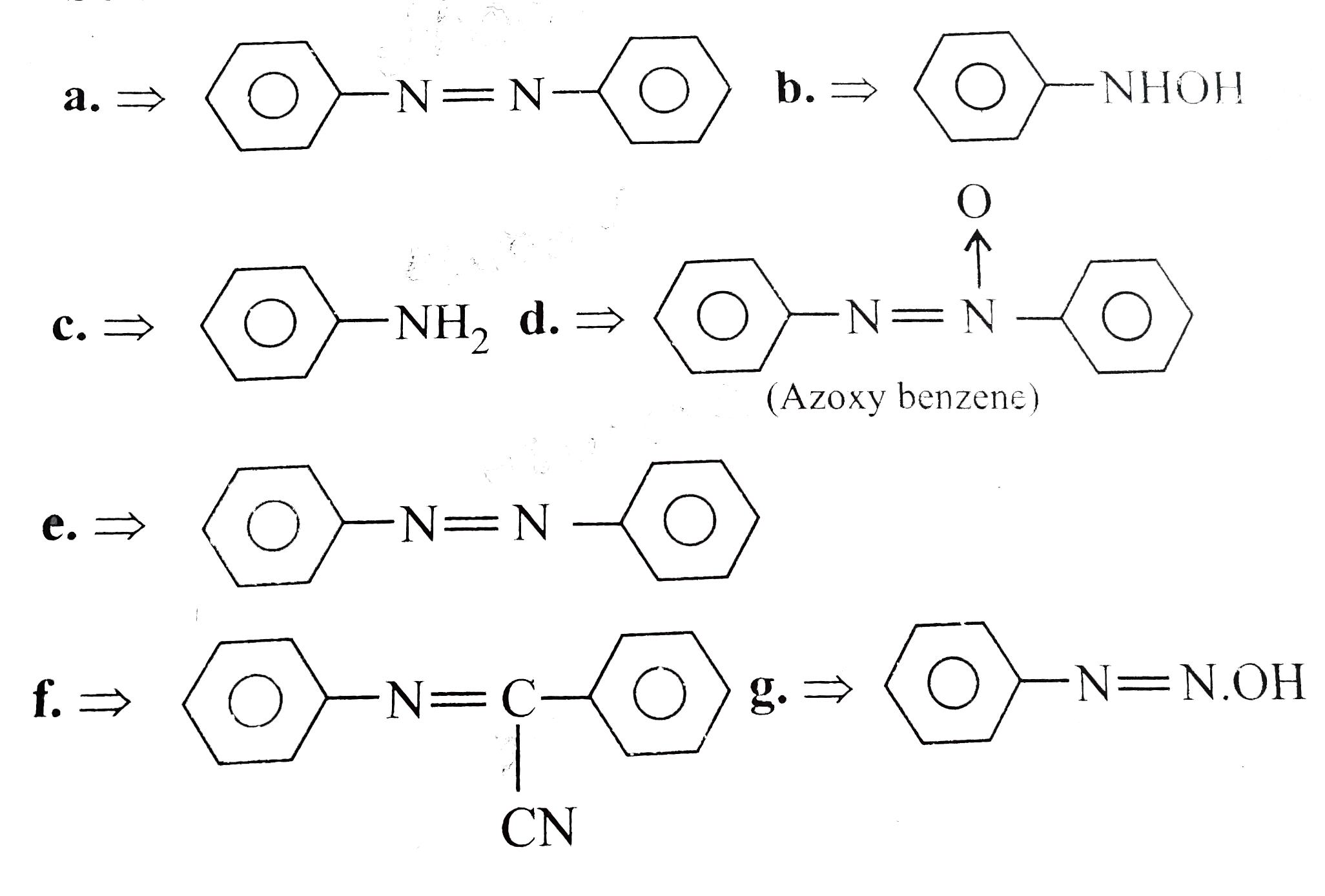
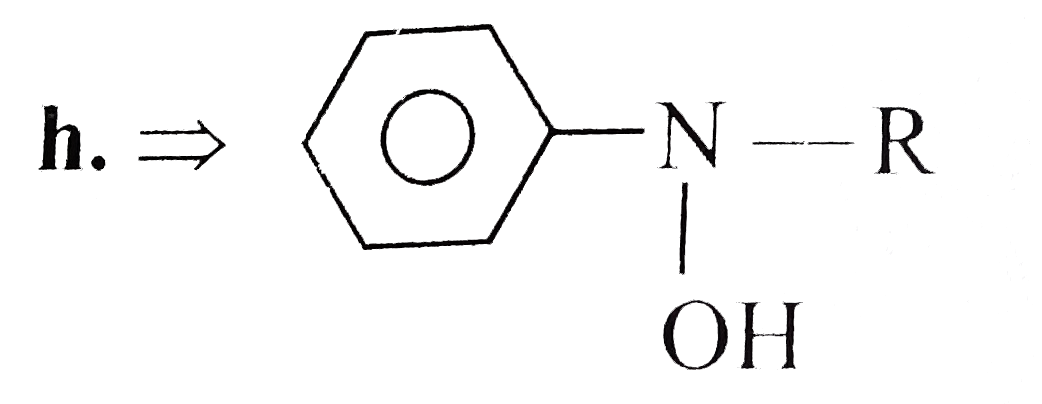
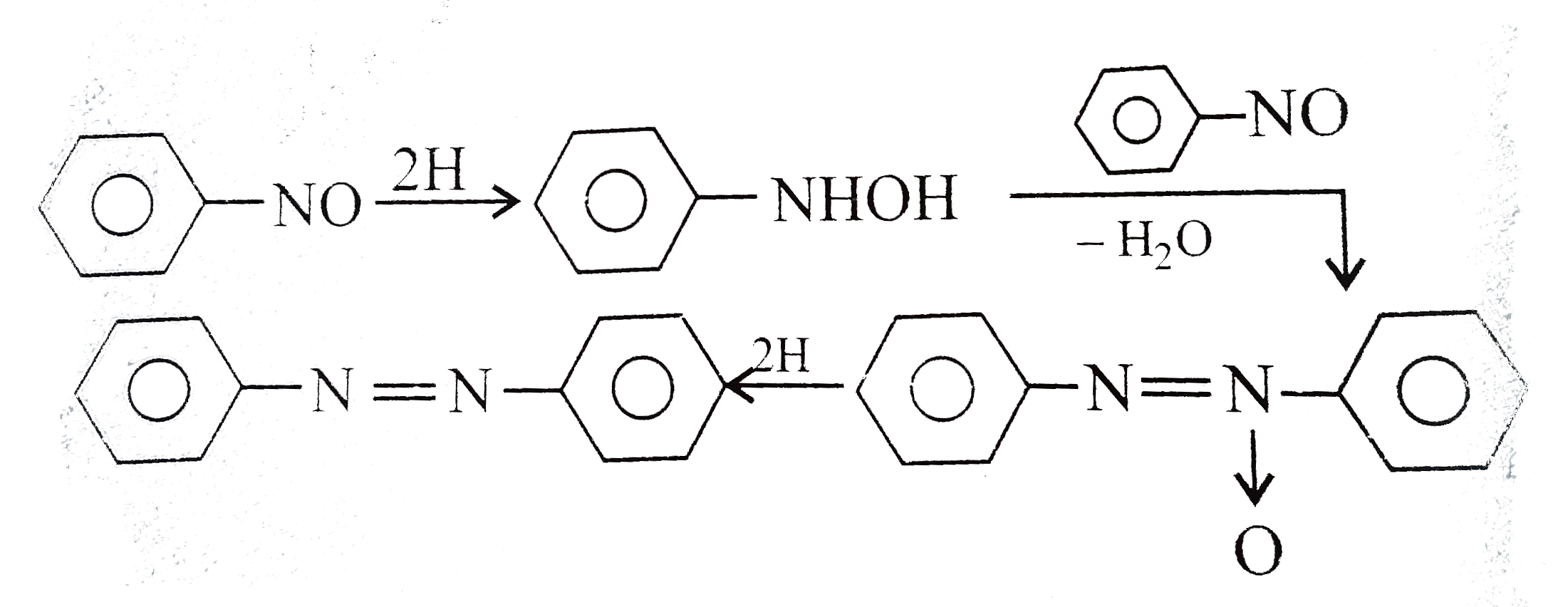
Text Solution
Verified by Experts
|
Topper's Solved these Questions
REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercise|11 VideosView PlaylistREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercise (Linked Comprehension)|52 VideosView PlaylistREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Solved Example|6 VideosView PlaylistQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 VideosView PlaylistSOLID STATE
CENGAGE CHEMISTRY|Exercise Ex 1.2 (Objective)|9 VideosView Playlist
Similar Questions
Explore conceptually related problems