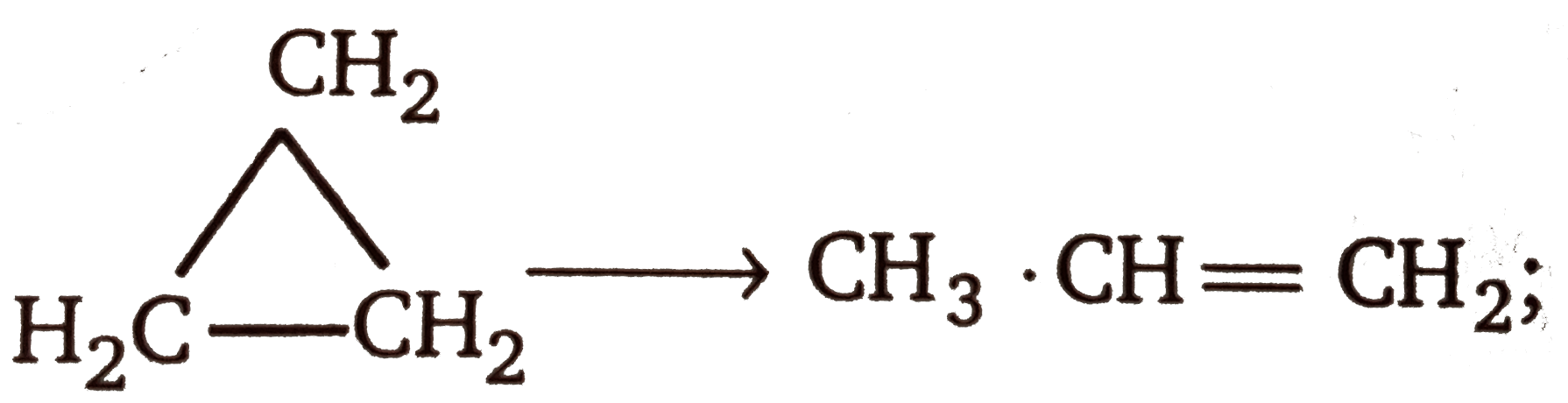
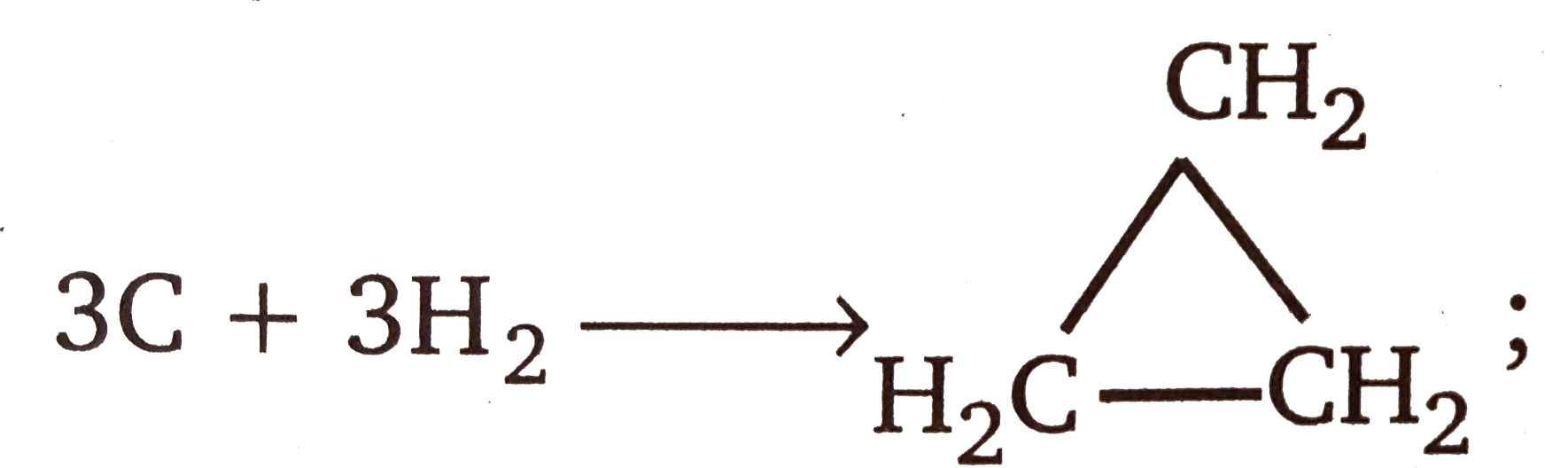
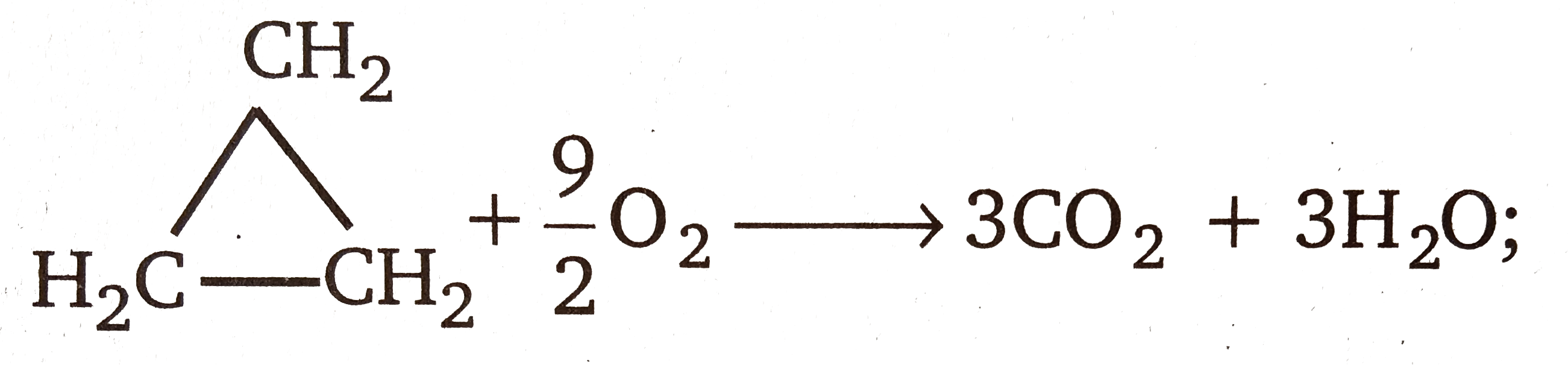Text Solution
Verified by Experts
Topper's Solved these Questions
THERMOCHEMISTRY
P BAHADUR|Exercise Exercise 9|1 VideosTHERMOCHEMISTRY
P BAHADUR|Exercise Exercise 10|1 VideosTHERMOCHEMISTRY
P BAHADUR|Exercise Exercise 7|1 VideosREDOX REACTIONS
P BAHADUR|Exercise Exercise(8) Statement:Explanation type problems|19 VideosTHERMODYNAMICS
P BAHADUR|Exercise Exercise|232 Videos
Similar Questions
Explore conceptually related problems