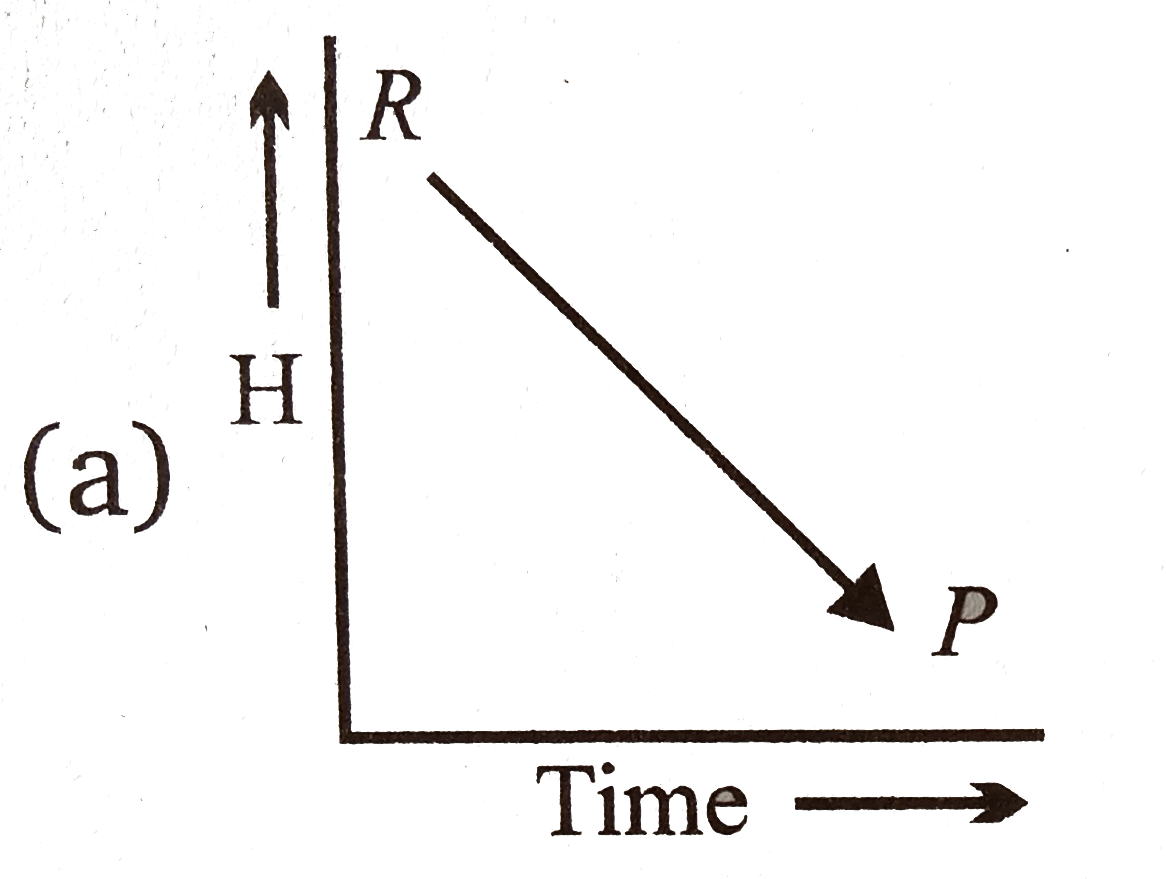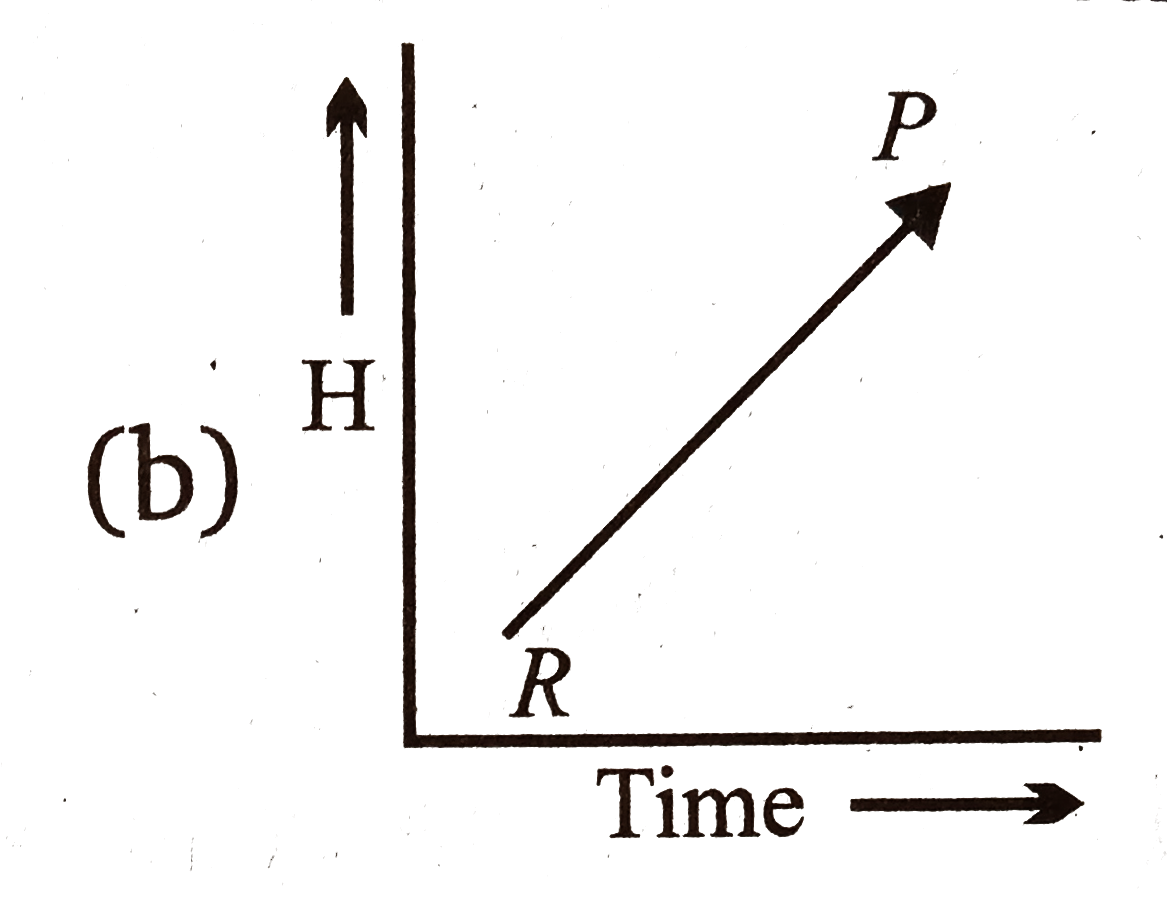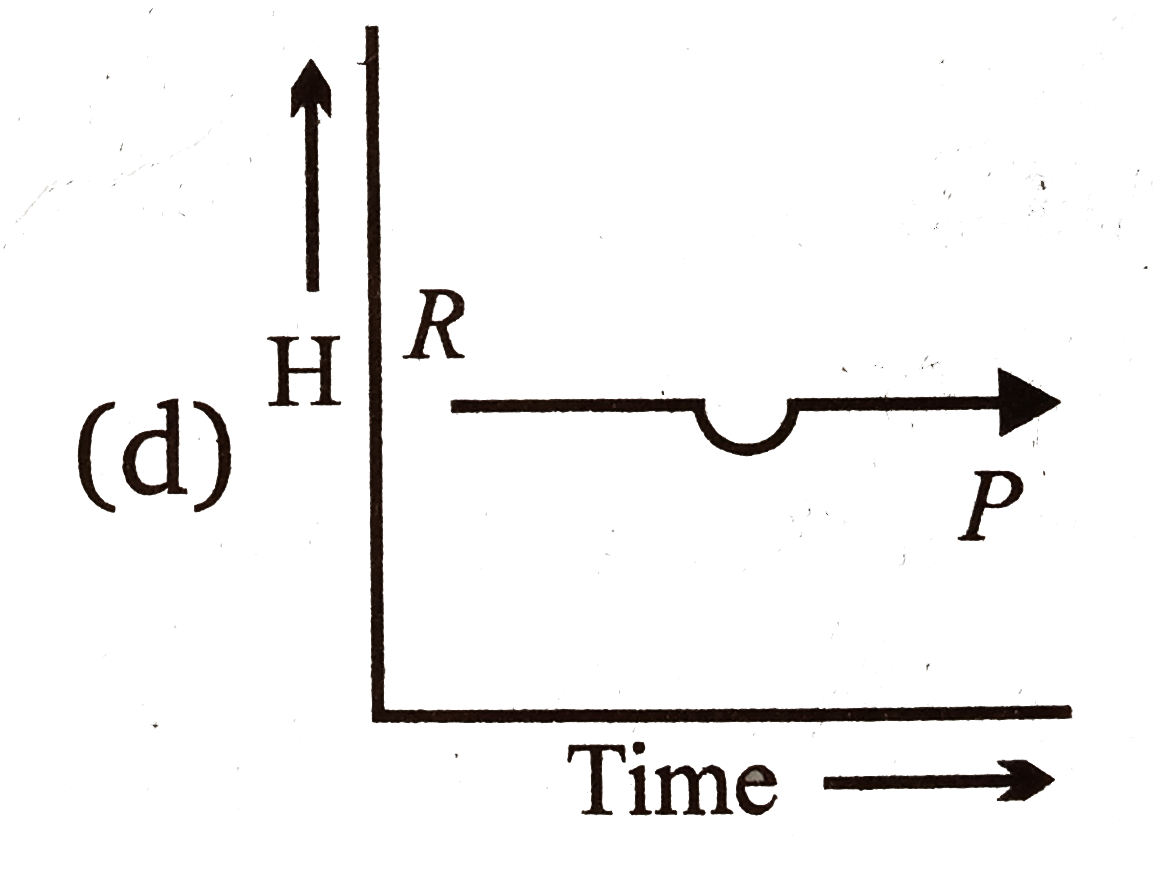A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
THERMOCHEMISTRY
P BAHADUR|Exercise Exercise 3B objective problems|18 VideosTHERMOCHEMISTRY
P BAHADUR|Exercise Exercise 4 objective Problems|21 VideosTHERMOCHEMISTRY
P BAHADUR|Exercise Exercise 27|1 VideosREDOX REACTIONS
P BAHADUR|Exercise Exercise(8) Statement:Explanation type problems|19 VideosTHERMODYNAMICS
P BAHADUR|Exercise Exercise|232 Videos
Similar Questions
Explore conceptually related problems
P BAHADUR-THERMOCHEMISTRY-Exercise 3a objective problems
- The compound with negative value of heat of formation are called :
Text Solution
|
- Which reaction is endothermic in nature ?
Text Solution
|
- Which plot represents for an exothermic reaction ?
Text Solution
|
- For the reaction ,3O(2)rarr 2O(3),DeltaH=+ve. We can say that :
Text Solution
|
- The enthalpy changes of formation of the gaseous oxides of nitrogen (N...
Text Solution
|
- The heats of neutralisation of four acidsA,B,C and D are -13.7,-9.4,-1...
Text Solution
|
- Which fuel provides the highest calorific values ?
Text Solution
|
- In a flask, colourless N(2)O(4) is in equilibrium with brown-coloured ...
Text Solution
|
- The difference between the heats of reaction at constant pressure and ...
Text Solution
|
- The temperature of a 5mL of strong acid increases by 5^(@) when 5 mL o...
Text Solution
|
- Heat of combustion of CH(4),C(2)H(4),C(2)H(6) are -890,-1411 and -1560...
Text Solution
|
- In which case of mixing of a strong acid and a base each of 1N concent...
Text Solution
|
- The dissociation energy of CH(4) and C(2)H(6) are respectively 360 and...
Text Solution
|
- Given that , A(s) rarr A(l)DeltaH=x A(l) rarr A(g), DeltaH=y The...
Text Solution
|
- A person requires 2870kcal of energy to lead normal daily life. If hea...
Text Solution
|
- If, combustion of 4g of CH(4) liberates 2.5kcal of heat, the heat of c...
Text Solution
|
- A solution of 500mL of 2M KOH is added to 500mL of 2M HCl and the mixt...
Text Solution
|
- Under the same conditions, how many mL of 1M KOH and 0.5M H(2)SO(4)sol...
Text Solution
|
- AB,A(2) and B(2) are diatomic molecules. If the bond enthalpies of A(2...
Text Solution
|
- DeltaC(p) for change ,N(2(g))+3H(2(g))=2NH(3(g)) is :
Text Solution
|