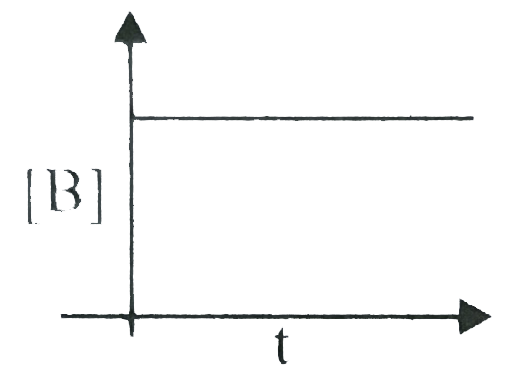
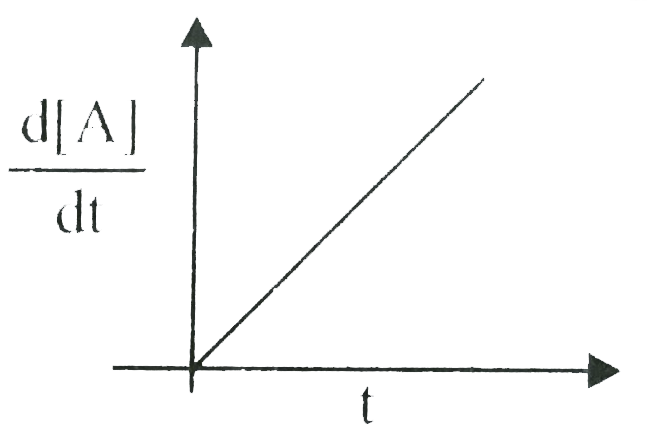
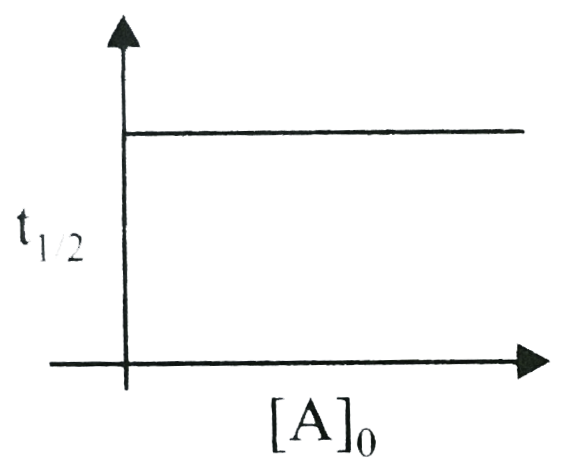
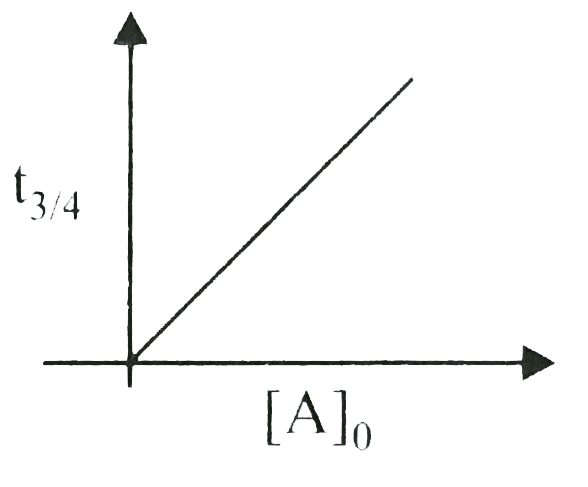
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
A2Z|Exercise Initial Rate Method And Ostwaid Method|18 VideosCHEMICAL KINETICS
A2Z|Exercise Arrhenius Equation, Effect Of Temprature And Effect Of Catalysts|35 VideosCHEMICAL KINETICS
A2Z|Exercise Rate Law, Law Of Mass Action, Order Of The Reaction, And Molecularity|48 VideosBIOMOLECULES
A2Z|Exercise Section D - Chapter End Test|30 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL KINETICS-Integrated Rate Expression
- 99% of a first order reaction was completed in 32 min. When will 99.9%...
Text Solution
|
- For which order reaction a straight line is obtained along with x-axis...
Text Solution
|
- Which graph represents zero-order reaction [A(g) rarr B (g)] ?
Text Solution
|
- ……………………… reaction obeys the expresison t(1//2) = 1//ka in chemical ki...
Text Solution
|
- t(1//4) can be taken as the time taken for concentration of reactant t...
Text Solution
|
- A reaction that is of the first order with respect to reactant A has a...
Text Solution
|
- A first-order reaction was started with a decimolar solution of the re...
Text Solution
|
- A first order reaction is half completed in 45 minutes. How long does ...
Text Solution
|
- The rate constant of reaction 2 A + B rarr C is 2.57 xx 10^(-5) L "...
Text Solution
|
- Decay constant of a reaction is 1.1 xx 10^(-9)//sec, then the half-lif...
Text Solution
|
- A follows first order reaction. (A) rarr Product The concentration...
Text Solution
|
- Gaseous N(2)O(5) decomposes according to the following equation: N(2...
Text Solution
|
- Which of the following curve represent zero order reaction of A rarr p...
Text Solution
|
- t(1//2) = constant confirms the first order of the reaction as one a^(...
Text Solution
|
- In presence of HCl, sucrose gets hydrolysed into glucose and fructose....
Text Solution
|
- Consider a reaction A(g) rarr 3B(g) + 2C(g) with rate constant is 1.38...
Text Solution
|
- For the reaction A + 2B rarr products (started with concentration take...
Text Solution
|
- A reaction 2A + B overset(k)rarr C+ D is first order with respect to A...
Text Solution
|
- The time for half-life period of a certain reaction, A rarr products i...
Text Solution
|
- What will be the order of reaction and rate constant for a chemical ch...
Text Solution
|