A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
A2Z|Exercise Parallel Reaction, Consecutive Reaction, Special Cases Of First-Order Reactions|25 VideosCHEMICAL KINETICS
A2Z|Exercise Section B - Assertion Reasoning|21 VideosCHEMICAL KINETICS
A2Z|Exercise Initial Rate Method And Ostwaid Method|18 VideosBIOMOLECULES
A2Z|Exercise Section D - Chapter End Test|30 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL KINETICS-Arrhenius Equation, Effect Of Temprature And Effect Of Catalysts
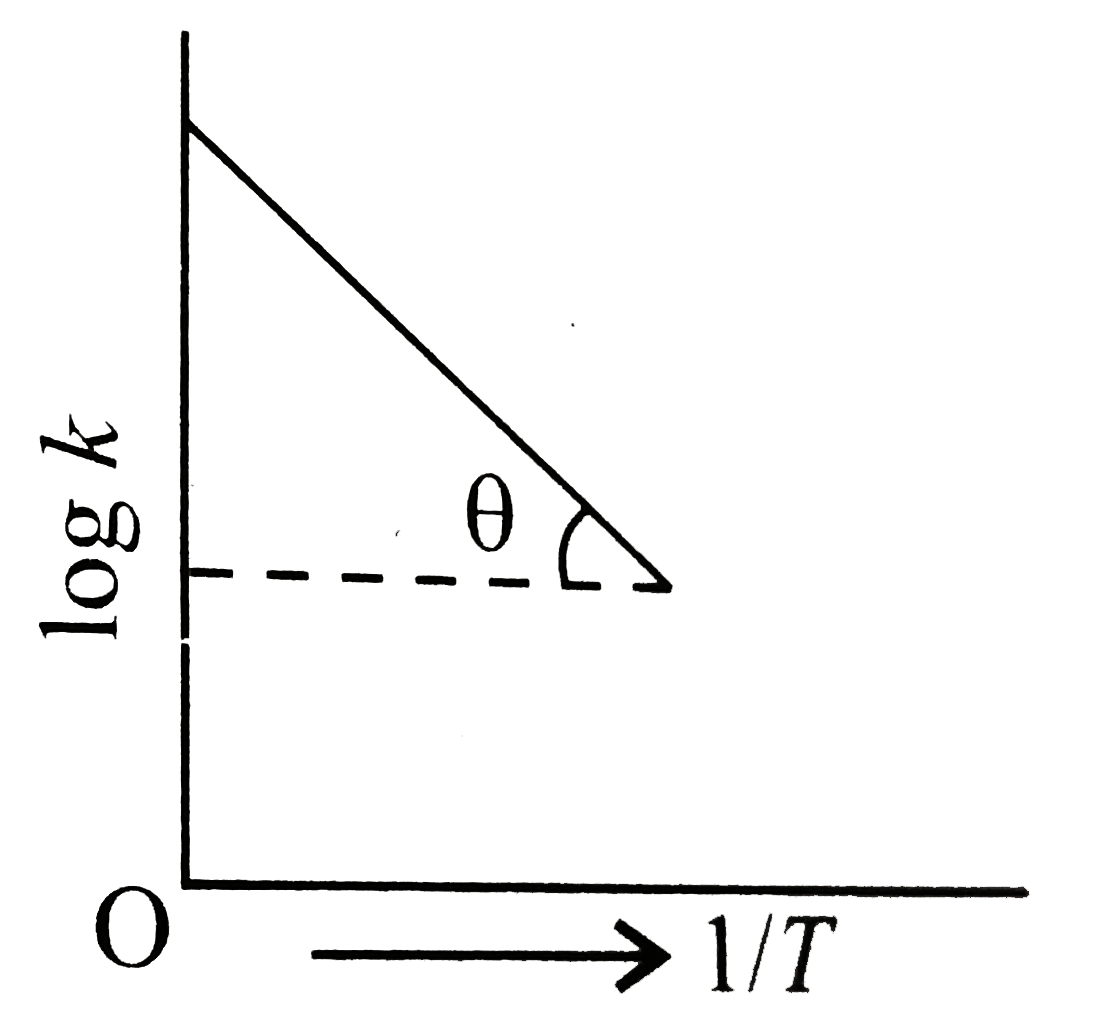
- Graph between log k and 1//T [k rate constant (s^(-1)) and T and the t...
Text Solution
|
- Rate of which reactions increases with temperature:
Text Solution
|
- Which of the following statement is not true according to collision th...
Text Solution
|
- The activation energies of two reactions are E(a1) and E(a2) with E(a1...
Text Solution
|
- Which one is correct for K = A e^(-E(a)//RT ?
Text Solution
|
- The slope of the line graph of log k versus 1//T for the reaction N(2)...
Text Solution
|
- For a zero order reaction. Which of the following statement is false:
Text Solution
|
- A large increase in the rate of a reaction for a rise in temperature i...
Text Solution
|
- The rate constant (K') of one reaction is double of the rate constant ...
Text Solution
|
- The energy of activations for forward and backward change for an endot...
Text Solution
|
- The maximum value of activation energy is equal to:
Text Solution
|
- The rate constant, the activation energy, and the Arrhenius parameter ...
Text Solution
|
- Which is incorrect from the theory of Arrhenius's equation ?
Text Solution
|
- Rate of a reaction can be expressed by Arrhenius equation as: k = Ae...
Text Solution
|
- The Delta H value of the reaction H(2)+Cl(2) hArr 2HCl is -44.12 kcal....
Text Solution
|
- The Activation energy for a chemical reaction mainly depends upon
Text Solution
|
- A molecule of gas is struck by another molecule of the same gas, the f...
Text Solution
|
- Activation energy of a chemical reaction can be determined by
Text Solution
|
- The energies of activation for forward and reverse reaction for A(2)+...
Text Solution
|
- ArarrB, DeltaH= -10 KJ mol^(-1), E(a(f))=50 KJ mol^(-1), then E(a) of ...
Text Solution
|