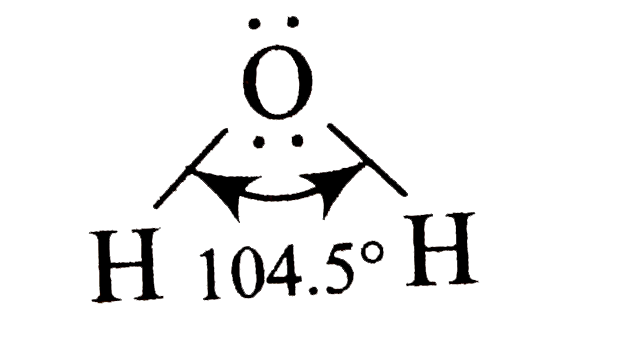A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 6|7 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 7|12 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 4|15 VideosAROMATIC HYDROCARBONS
R SHARMA|Exercise Archives|59 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise ARCHIVES|37 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Follow-up Test 5
- Which of the following molecules has the longest nitrogen-nitrogen bon...
Text Solution
|
- Which of the following molecules has the maximum bond enthalpy?
Text Solution
|
- Which of the following molecules has the highest value of carbon-carbo...
Text Solution
|
- Which of the following has the shortest bond length?
Text Solution
|
- Which of the following bonds has the lowest bond enthalpy?
Text Solution
|
- In ethene, the carbon-carbon bond distance is
Text Solution
|
- The H-O-H bond angle in water is
Text Solution
|