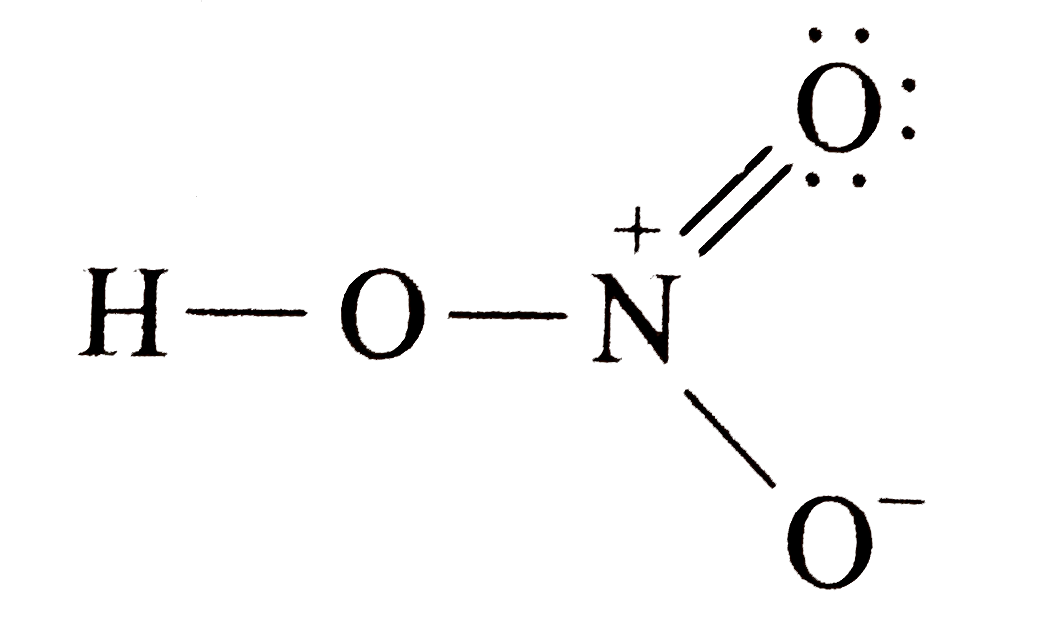
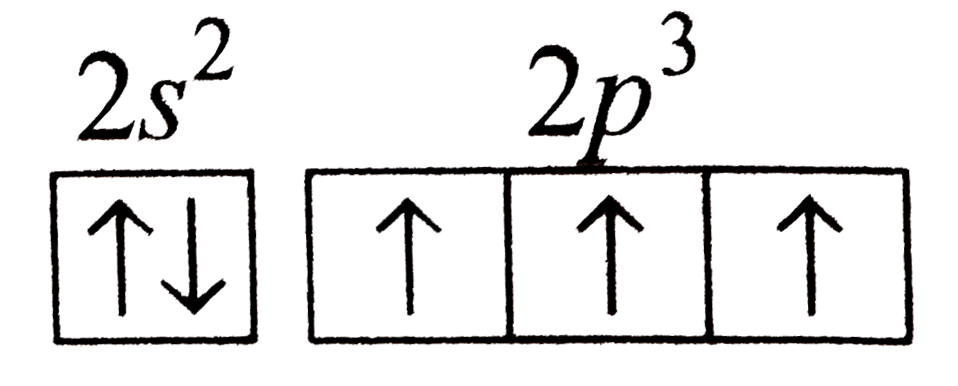A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 5|7 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 6|7 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 3|8 VideosAROMATIC HYDROCARBONS
R SHARMA|Exercise Archives|59 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise ARCHIVES|37 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Follow-up Test 4
- The nitrogen atom shows a maximum covalency of
Text Solution
|
- Carbon suboxide (C3O2) is a foul-smelling gas. Which of the following ...
Text Solution
|
- BF3 and NH3 combine readily because of the formation of
Text Solution
|
- Lewis formulas are not normally written for compounds containingelemen...
Text Solution
|
- Which of the following is an electron-deficient compound?
Text Solution
|
- Which one of the following is not a hypervalent compound?
Text Solution
|
- Which of the following compounds does not follow the octet rule?
Text Solution
|
- Which of the following compound contains ionic as well as covalent bon...
Text Solution
|
- Maximum covalency shows by phosphorous is
Text Solution
|
- In the linear I3^(-) (triiodide ion), the central iodine atom contains
Text Solution
|
- In the Lewis structure of acetic acid, there are
Text Solution
|
- Which of the following types of bonds are present in N2O5? (i) Ionic...
Text Solution
|
- The formal charges on the three O atoms in the O3 molecule are
Text Solution
|
- Which of the following is the most likely Lewis structure of nitrosyl ...
Text Solution
|
- In allene, C3H4, three C atoms are joined by
Text Solution
|