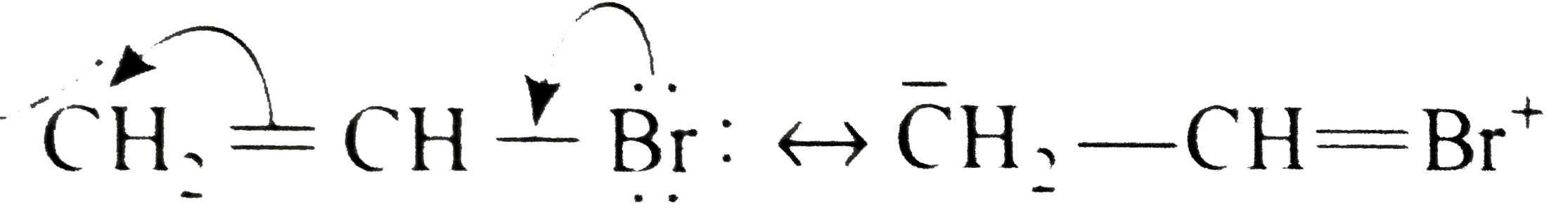A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALKYNES-Follow-up Test 4
- Which of the following hydrocarbons are most polar in nature?
Text Solution
|
- What is the minimum number of C atoms required to have a carbon-corbon...
Text Solution
|
- CH3CH=CH2underset(C Cl4)overset(Br2)tounderset(excess)overset(NaNH2)to...
Text Solution
|
- Roverset(PCl5)toAunderset(excess)overset(NaNH2)toBoverset(H2O)topropyn...
Text Solution
|
- 2,3-Dibromobuteneoverset(Reag ent)tobut-2-yne Identify the reagent.
Text Solution
|
- CH3C-=Choverset(NaNH2)toAoverset(CH3CH2Br)toB Identify the product B...
Text Solution
|
- Sodium propynide reacts with 2-bromo-2-methylpropane The major product...
Text Solution
|
- CH3C-=Choverset(CH3MgBr)toAoverset(CH3CH2Br)toB The final product B ...
Text Solution
|
- overset(PCl5)toAoverset(3NaNH2)toB The final product B of the reacti...
Text Solution
|
- How many moles of sodamide are required to synthesize and alkyne from ...
Text Solution
|