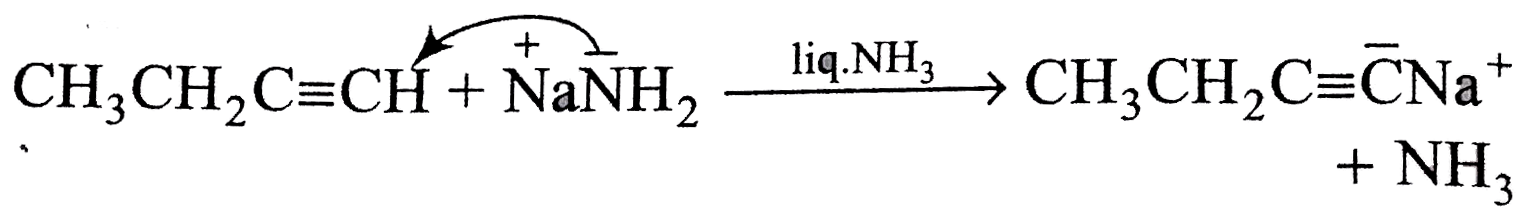A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALKYNES-Archives
- From which one of the following can both ethylene and acetylene be pre...
Text Solution
|
- The treatment of CH(3)MgX with CH-=CH produces
Text Solution
|
- The hydrocarbon which can react with sodium in liquid ammonia is
Text Solution
|
- Base strength of (i) CH3CH2^(-) (ii) CH2=CH^(-) (iii) CH-=C^(-)
Text Solution
|
- The product C is CH(3).CH(2).C-=CH+HCl rarrBoverset(HI)rarrC
Text Solution
|
- Under which of the following conditions does the reaction HC-=CH+CH2OH...
Text Solution
|
- Which of the following reactions will yeild 2,2-dibromo propane?
Text Solution
|
- The reaction of acetylene and propyne with HgSO4 in the presence of H2...
Text Solution
|
- An organic compound decolorizes Br2 water and also gives red ppt. with...
Text Solution
|
- Ethene and ethyne can be distinguished by
Text Solution
|
- In the following sequency of reactions CH3CH2Broverset(KOH)toXoverse...
Text Solution
|
- HC-=CH reacts with acetic acid in the presence of Hg^(2g+) ions to giv...
Text Solution
|
- Aoverset(H(2)//"Lindlar's catalyst")larrCH3C-=C CH(3)overset(Na//Liq" ...
Text Solution
|
- In the reaction CH3C-=C CH3underset((Zn)/(H2O))overset(R)toCH3COCOCH...
Text Solution
|
- The products of the following reactions CH3C-=C CH2CH3underset("oxid...
Text Solution
|
- Propyne when passed through a hot iron tube at 400^@C produces
Text Solution
|
- H--=-Hoverset((i)O3)underset((ii)(H2O)//(Zn))rarr(A)overset(Zn//CH3COO...
Text Solution
|
- Which of these will not react with acetylene?
Text Solution
|
- When CH3CH2CHCl2 is treated with NaNH2, the product formed is
Text Solution
|
- Acetylene polymerizes in the presence of Ni(CN)2 under pressure to for...
Text Solution
|