A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
R SHARMA-ALKENES-Follow-up-10
- Markovnikov's rule is empirical but may be explained theoretically on ...
Text Solution
|
- Alkanes are absorbed by concentrated sulphuric acid to from
Text Solution
|
- Alkyl hydrogen sulphates can be easily hydrolyzed to alcohols by heati...
Text Solution
|
- Which of the following is not the characterstic of acid catalyzed hydr...
Text Solution
|
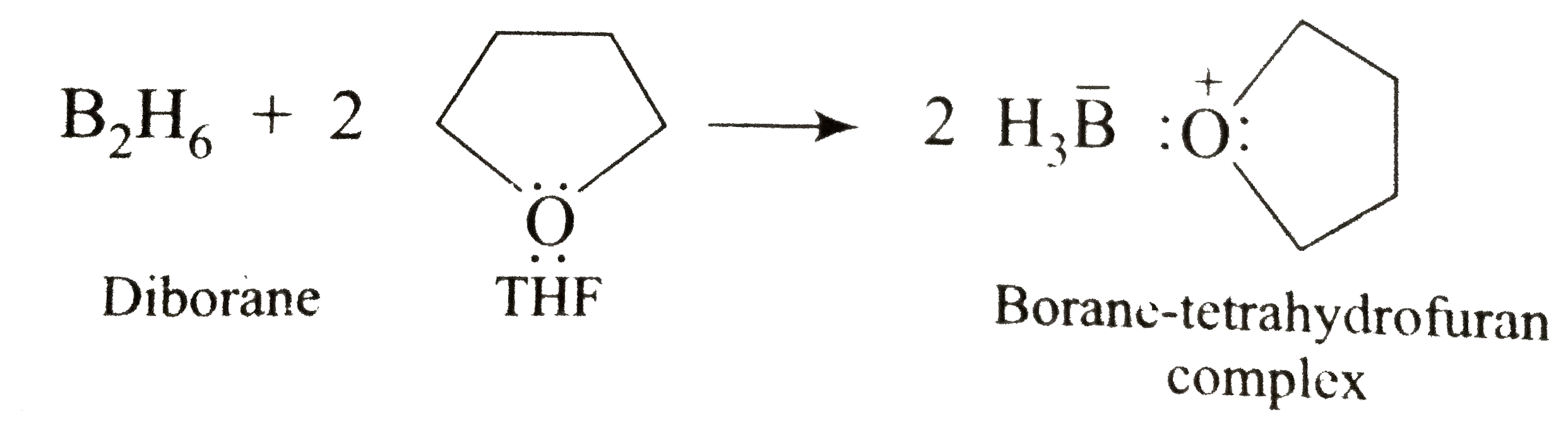
- The addition of the compound containing a hydrogen-boron bond H-B lt(c...
Text Solution
|
- Propene is allowed to react with diobrane and the product is treated w...
Text Solution
|
- Consider the reaction. The product formed is
Text Solution
|
- In the reaction CH(3)CH=CH(2)underset((2)NaBH(4))overset((1)(CH(3)CO...
Text Solution
|
- In the reaction CH(3)CH(2)CH=CH(2)underset((2)NaBD(4))overset((1)Hg(...
Text Solution
|
- Which of the following is formed when three moles of propene react wit...
Text Solution
|
- The electrophile in hydrocarbon is
Text Solution
|
- CH(3)CH=CH(2)underset(THF)overset(B(3)H(6))rarrAoverset(Br(2))rarrB ...
Text Solution
|