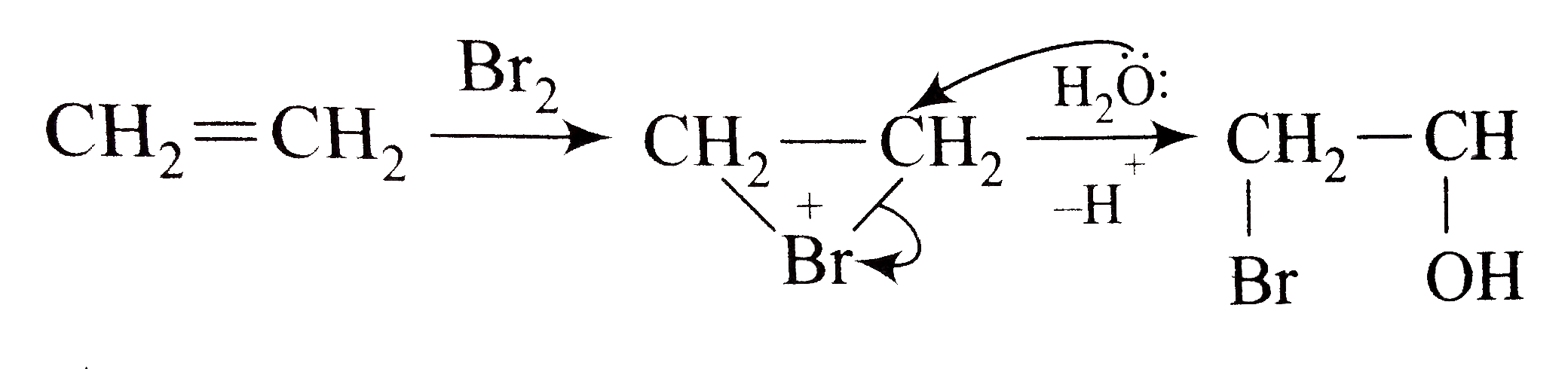A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALKENES-Follow-up-11
- Ethene on reaction with Br(2) in H(2)O forms mainly
Text Solution
|
- The intermediate formed in the reaction CH(2)=CH(2)+HOCloverset(H^(+...
Text Solution
|
- The product formed in the reaction CH(3)-underset(CH(3))underset(|)C...
Text Solution
|
- The minor product obtained in the acid-catalyzed dimerization of methy...
Text Solution
|
- (CH(3))(2)C=CH(2)underset(H(2)O)overset(Br(2))rarr The major product...
Text Solution
|
- CH(3)CH=CH(2)+BrC Cl(3)overset("Peroxide")rarr The major product of ...
Text Solution
|
- CH(3)CH=CH(2)+NOClrarrP Identify the product.
Text Solution
|
- Consider the following reaction The product formed in the reactio...
Text Solution
|
- Ethylene reacts with CH(2)CI(2) in the presence of Zn-Cu couple to for...
Text Solution
|