A water cooler of storages capacity 120 liters can cool water at a constant rate of P watts. In a closed circulation system (as shown schematically in the figure), the water from the cooler is used to cool an external device that generates constantly 3kW of heat (thermal load). The temperature of water fed into the device cannot exceed `30^@C` and the entire stored 120 liters of water is initially cooled to `10^@C.` The entire system is thermally insulated. The minimum value of P ( in watts) for which the device can be operated for 3hours is
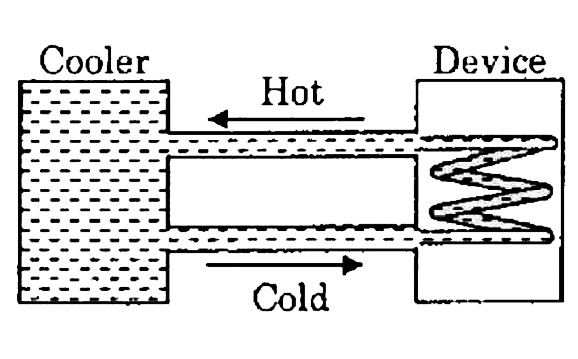
(Specific heat of water is `4.2kJkg^-1K^-1` and the density of water is `1000kgm^-3`)