Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise ADDITIONAL QUESTIONS (SHORT ANSWER QUESTIONS)|79 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise ADDITIONAL QUESTIONS (LONG ANSWER QUESTIONS)|7 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (LONG ANSWER QUESTIONS)|3 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|22 VideosP-BLOCK ELEMENTS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|25 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ORGANIC COMPOUNDS CONTAINING NITROGEN-ADDITIONAL QUESTIONS (VERY SHORT ANSWER QUESTIONS)
- (a) Arrange the following in the increasing order of boiling points: ...
Text Solution
|
- Account for the following (i)Primary amines (R-NH(2)) have higher b...
Text Solution
|
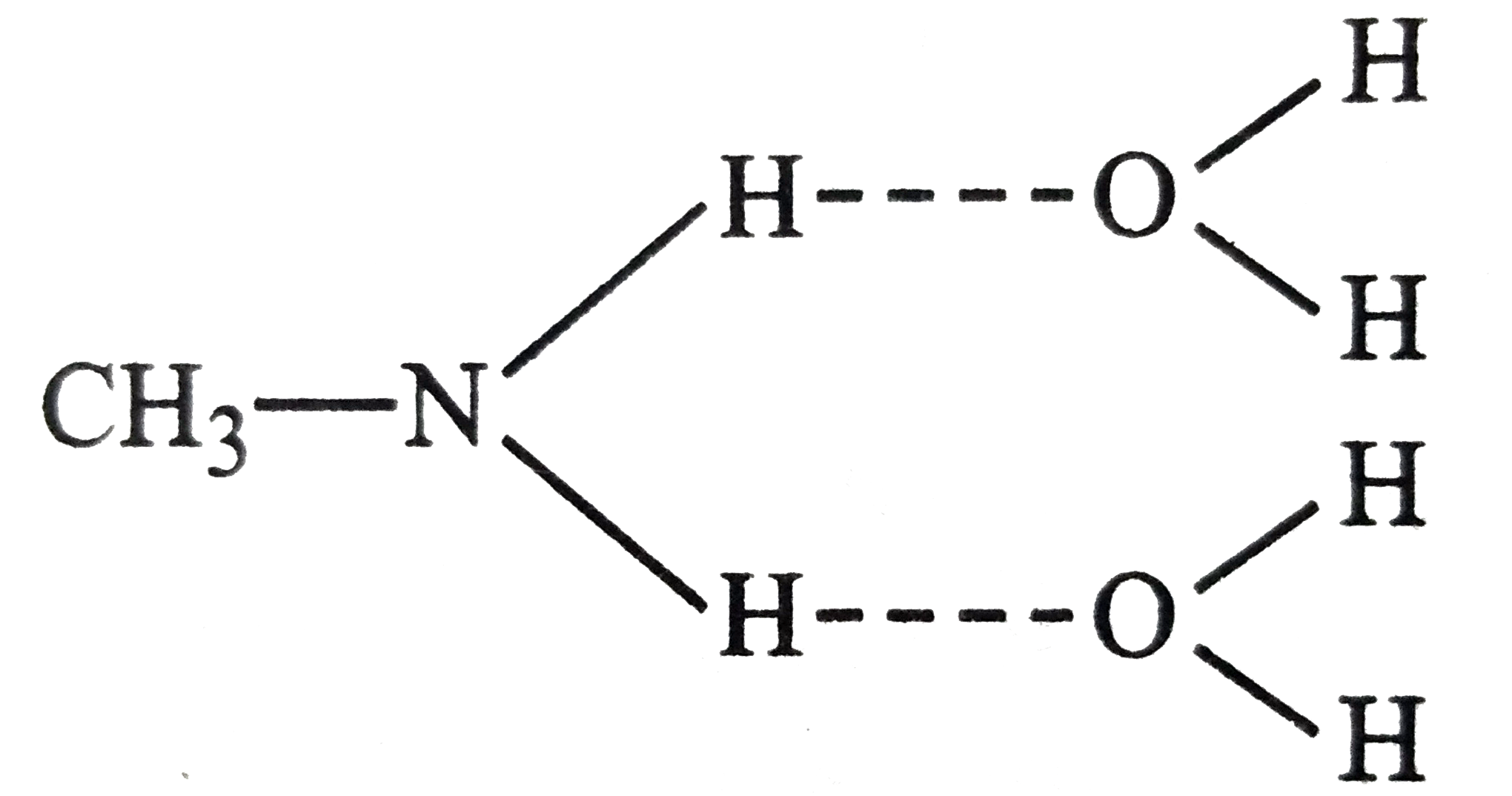
- Methylamine is soluble in water but not aniline. Explain.
Text Solution
|
- Arrange the following compunds in increasing order of solubility in wa...
Text Solution
|
- What amine salts are used for determining their molecular masses?
Text Solution
|
- Why is carbon nitrogen bond length in aromatic amines shorter than in ...
Text Solution
|
- Why is an alkylamine more basic than ammonia ?
Text Solution
|
- Which is more acidic (or basic), aniline or ammonia?
Text Solution
|
- Arrange the following in decreasing order of their basic strength: amm...
Text Solution
|
- How is the basic strength of aromatic amines affected by the presence...
Text Solution
|
- Arrange the following in the increasing order of their basicities. (...
Text Solution
|
- Arrange the following in increasing order of their acid strength: meth...
Text Solution
|
- CH(3)CONH(2) is a weaker base than CH(3)CH(2)NH(2).
Text Solution
|
- Give the structures of the products A, B and C in the following reacti...
Text Solution
|
- How will you canvert the following : (i) Nitrobenzene into aniline, ...
Text Solution
|
- The product of mustard oil reaction is
Text Solution
|
- Give a chemical test to distinguish between a primary and a secondary ...
Text Solution
|
- Electrophilic substitution in case of aromatic amines takes place more...
Text Solution
|
- Direct nitration of aniline is not carried out. Explain.
Text Solution
|
- Give reason: Aniline gets coloured on standing in air for a long time...
Text Solution
|