Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES
RESONANCE|Exercise Ex-3(IIT-JEE)Part-II|15 VideosBIOMOLECULES
RESONANCE|Exercise Ex-3(IIT-JEE)Part-III|34 VideosBIOMOLECULES
RESONANCE|Exercise Ex-2(Comprehension)Part-IV|5 VideosBASIC CONCEPTS
RESONANCE|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosBIOMOLECULES & POLYMER
RESONANCE|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|33 Videos
RESONANCE-BIOMOLECULES-Ex-3(IIT-JEE)Part-I
- Aspartame, an artificial sweetener, is peptide and has the following s...
Text Solution
|
- Following two amino acids lysine and glutamine form dipeptide linkage....
Text Solution
|
- Write down the heteroheneous catalyst involved in the polymerisation o...
Text Solution
|
- Which of the following pair give positive Tollen's Test ?
Text Solution
|
- The Fischer projection formula of D-glucose is (i) Give Fischer p...
Text Solution
|
- The two forms of D-glucopyranose obtained from solution of D-glucose a...
Text Solution
|
- Which of the following disaccharide will not reduce tollen's reagent. ...
Text Solution
|
- Statement I: Glucose gives a reddish-brown precipitate with fehling's ...
Text Solution
|
- Cellulose upon acetylation with excess anhydride//H(2)SO(4) (catalytic...
Text Solution
|
- Among cellulose, poly viny chloride), nylon and nutural rubber, the po...
Text Solution
|
- The correct statement(s) about the following sugars (X) and (Y) is//ar...
Text Solution
|
- A decapeptide (Mol. Wt. 769) on complete hydrolysis gives glycine (Mol...
Text Solution
|
- The following carbohydrate is:
Text Solution
|
- The correct functional group X and the reagent//reaction conditions Y ...
Text Solution
|
- When the following aldohexose exists in its D-configuration, the total...
Text Solution
|
- The substituents R(1) and R(2) for nine peptides are listed in the tab...
Text Solution
|
- The total number of lone-pairs of electrons in melamine is.
Text Solution
|
- A tetrapeptide has -COOH group on alanine. This produces glycine (Gly)...
Text Solution
|
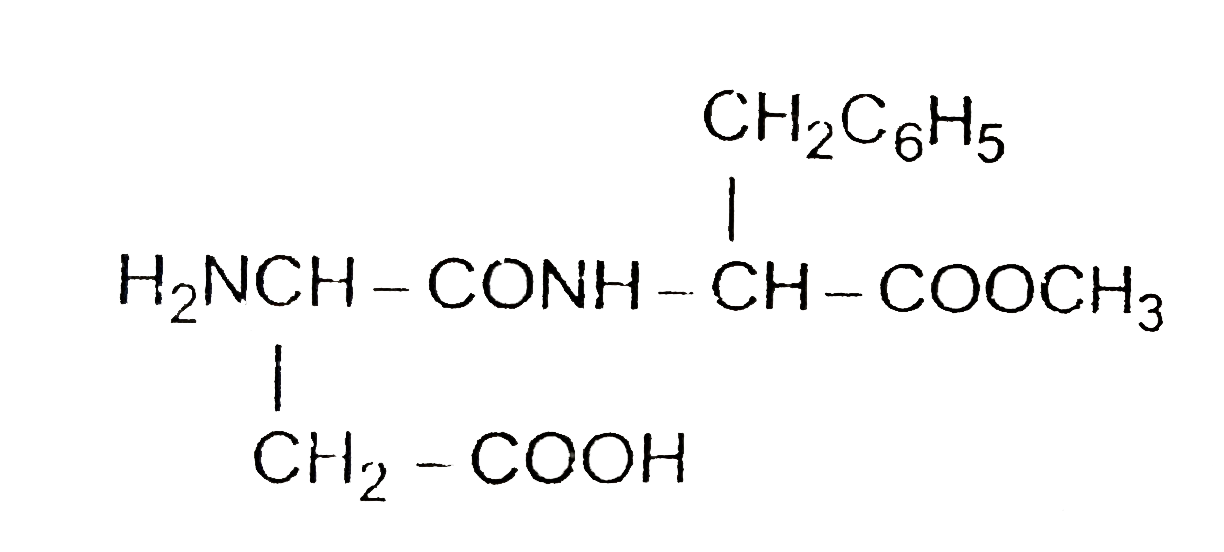 .
.