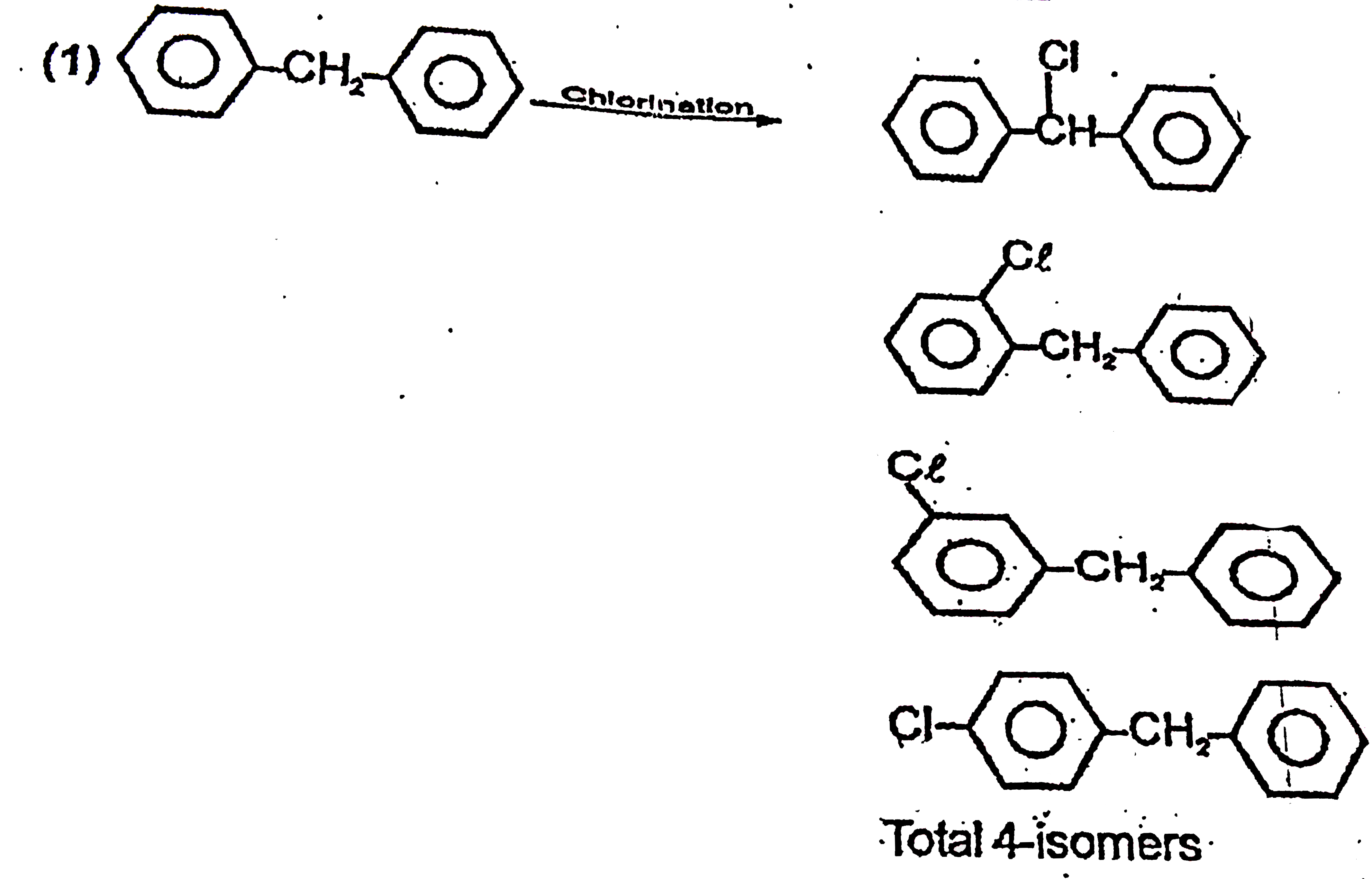A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-BASIC CONCEPTS-ORGANIC CHEMISTRY(BASIC CONCEPTS)
- The molecular formula of diphenyl methane, is C(13)H(12). How many ...
Text Solution
|
- Which of the following hydrocabon can give only acetone and CO(2)on oz...
Text Solution
|
- Which of the following IUPAC name is incorrect ?
Text Solution
|
- The general molecular formula , which represents the homologous series...
Text Solution
|
- The given structures are :
Text Solution
|
- The structure of isobutyl group in an organic compound is
Text Solution
|
- Which of the following compound is achiral ( optically inactive )?
Text Solution
|
- Which cannot show geometrical isomerism ?
Text Solution
|
- The following compounds can be distinguished by
Text Solution
|
- Total acyclic optically active isomers of C(3)H(2)D(2) are :
Text Solution
|
- Identify the compound and find the relation between them .
Text Solution
|
- Correct IUPAC name of the compound is NH(2)-underset(HOOC)underset(|...
Text Solution
|
- How many structural isomers containing a benzene ring are possible for...
Text Solution
|
- Which of the following statement is incorrect ?
Text Solution
|
- Which of the following species can not exhibit geometrical isomerism ?
Text Solution
|
- The stereochemical formula of diastereomer 'Y' of optically active com...
Text Solution
|
- Which is correctly matched with IUPAC Name ?
Text Solution
|
- Which compound racemises ( looses optical activity ) due to tautomeris...
Text Solution
|
- In which compund D- exchange is possible in presence of OD^(-) // D(2)...
Text Solution
|
- Compound C(5)H(10) (A)underset((2) Me(2)S//H(2)O)overset((1)O(3))rarrB...
Text Solution
|
 is `C_(13)H_(12)`.
is `C_(13)H_(12)`.