Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS
RESONANCE|Exercise (MSPs)|7 VideosSOLUTIONS
RESONANCE|Exercise Board Level Exercise|28 VideosSOLUTION AND COLLIGATIVE PROPERTIES
RESONANCE|Exercise PHYSICAL CHEMITRY (SOLUTION & COLLIGATIVE PROPERTIES)|52 VideosSTEREOISOMERISM
RESONANCE|Exercise EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-SOLUTIONS-Advabced Level Problems (PART-2)
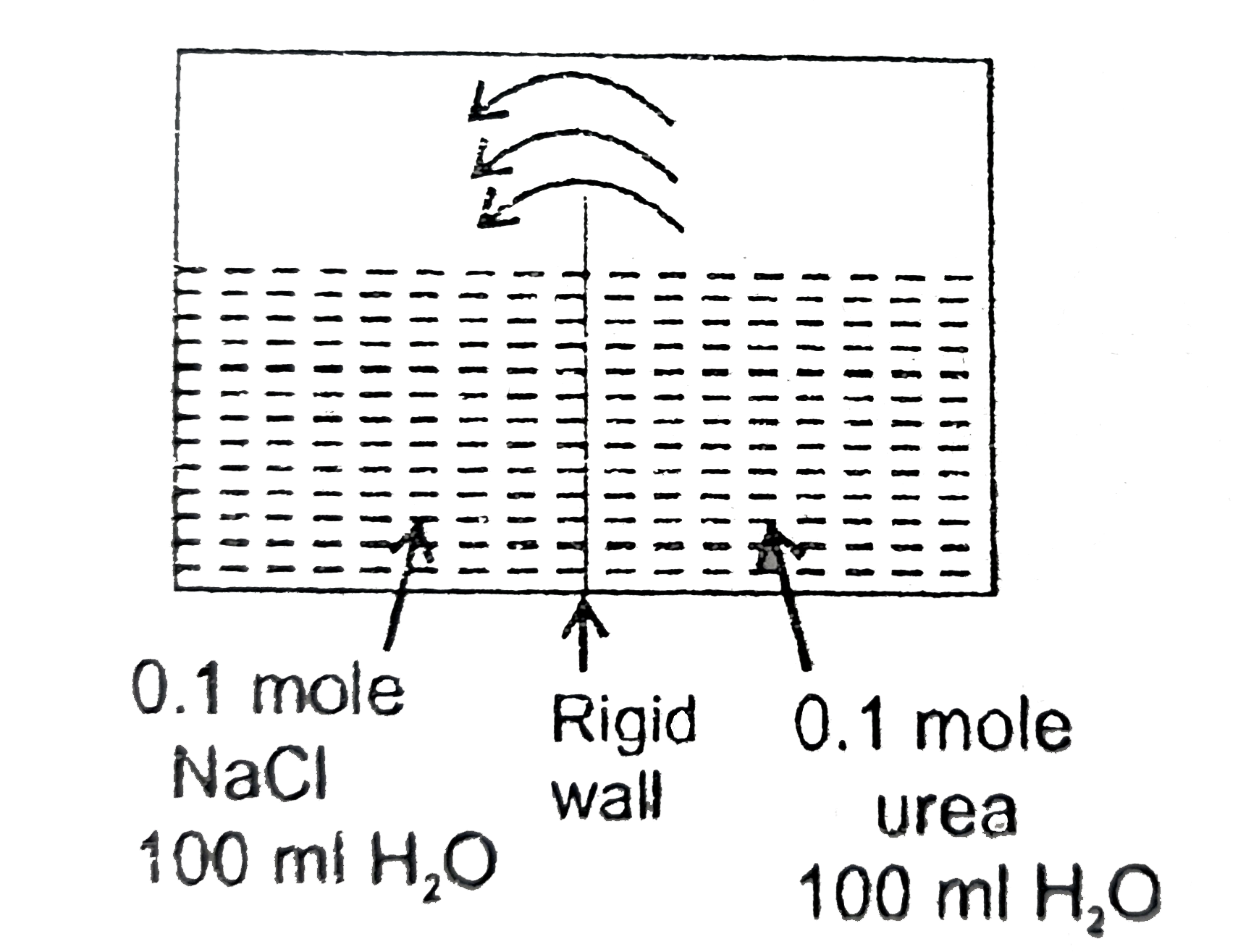
- What is the final volume of both container.
Text Solution
|
- A solution of 2.8g of Cdl(2) molar mass =364g "mol"^(-1)) in 20g water...
Text Solution
|
- Figure explains elevation in boiling point when a non-volatile solute ...
Text Solution
|
- It is found that elevation in boiling point of a given aq. NaCl soluti...
Text Solution
|
- There are two solutions each at 27^(@)C Solution A: contains 6g urea...
Text Solution
|
- How many grams of NaBr must be added to 270g of water to lower the vap...
Text Solution
|
- How many grams of sucrose must be added to 360g of water to lower the ...
Text Solution
|
- The vapour pressure of pure liquid A at 300K is 577 Torr and that of p...
Text Solution
|
- Phenol (C(6)H(5)OH) is found to exist as polymer. If there is 100% pol...
Text Solution
|
- Following are equimolal (=f equimolar) aqueous solutions (A) 1 m glu...
Text Solution
|
- The freezing point depression of 0.001 m K(x) [Fe(CN)(6)] is 7.10xx10^...
Text Solution
|
- The vapour pressure above a solution of 50g acetic acid (H(2)H(3)O(2)~...
Text Solution
|
- 1000 g of 1 molal aqueous solution of sucrose is cooled and maintaine...
Text Solution
|
- A certain solution of 1 m benzoic acid in benzene has a freezing point...
Text Solution
|
- An aqueous solution containing 288gm of a non-volatile compound havin...
Text Solution
|
- The freezing point of an aqueous solution of KCN containing 0.1892 mol...
Text Solution
|
- Sea water is found to contain 5.85% NaCI and9.50% MgCI(2) by weight of...
Text Solution
|
- Calculate the boiling point of water at 700mm pressure of Hg. The heat...
Text Solution
|
- Vapour pressure of C(6)H(6) and C(7)H(8) mixture at 50^(@)C is given b...
Text Solution
|
- An aqueous solution of glucose boils at 100.01^(@)C.The molal elevatio...
Text Solution
|
- Match the boiling point with K(b) for x,y and z, if molecular weight o...
Text Solution
|