A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-CHEMISTRY
- A gaseous mixture of ethene, ethane and methane having total volume 15...
Text Solution
|
- The polar as well as planar compound is
Text Solution
|
- What will be the correct IUPAC name of the following compound ?
Text Solution
|
- Which of the following options is not correct regarding the order of t...
Text Solution
|
- The maximum number of atoms in a plane in PCl(5) is
Text Solution
|
- IUPAC name of given compound is
Text Solution
|
- 1 gm -atom of nitrongen may represents
Text Solution
|
- Which of the ions does not exist ?
Text Solution
|
- In which of the following compounds there is absence of secondary carb...
Text Solution
|
- 150 ml of a solution containing 5 millimoles of A (special gravity =1....
Text Solution
|
- Consider the compound given below Hunderset(1)(C)-=underset(2)(C)-un...
Text Solution
|
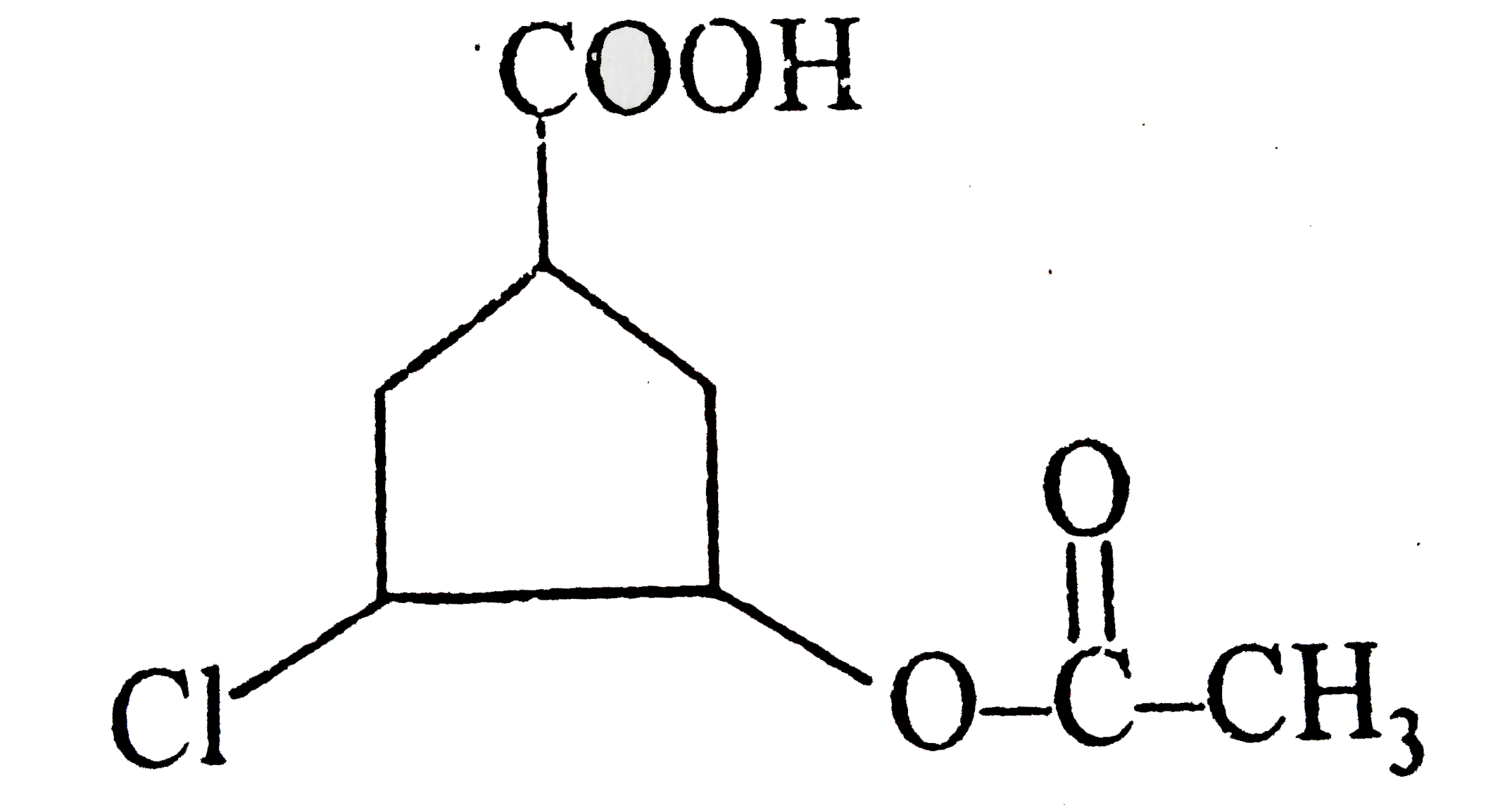
- The structure of 3-(Carboxymethyl) 5-ethanoyloxy cyclohexane carboxyli...
Text Solution
|
- When 20ml of mixture of O(2) and O(3) is heated the volume becomes 29m...
Text Solution
|
- The correct order of boiling point of the given compound is
Text Solution
|
- Which among following is correct IUPAC name ?
Text Solution
|
- Which of the following options is incorrect regarding Bohr's Model of ...
Text Solution
|
- Which of the following has maximum bond angle of anglexpx ?
Text Solution
|
- Give IUPAC name of following compound
Text Solution
|
- Which of the following is correct IUPAC name ?
Text Solution
|
- Each mole of substance A (Molar mass =720 ) required 10 moles of water...
Text Solution
|