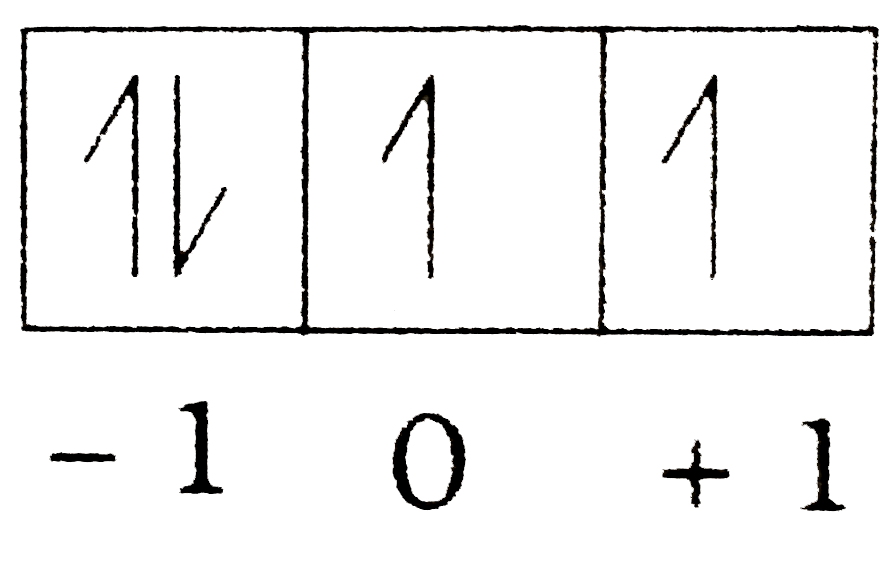Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - III 2 MARKS QUESTIONS )|38 VideosSTRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - IV 4 MARKS )|32 VideosSTRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - I 1/2 MARK QUESTIONS)|96 VideosREFLECTION AND REFRACTION
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|24 VideosSUMMATIVE ASSESSMENT
VGS PUBLICATION-BRILLIANT|Exercise SUMMATIVE ASSESSMENT|28 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-STRUCTURE OF ATOM -CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - II 1 MARK)
- Write the set of quantum numbers for the electrons in a 3p(z) orbital.
Text Solution
|
- What is the difference between an orbit and orbital ?
Text Solution
|
- Write the set of quantum numbers for the lastly entered electron of ox...
Text Solution
|
- What are the factors which influence electromagnetic energy '?
Text Solution
|
- Do all the p-orbitals have the same energy ?
Text Solution
|
- What is the value of Planck's constant
Text Solution
|
- What is maximum value of 'l' for n = 3 ?
Text Solution
|
- How many values can l have for n = 4 ?
Text Solution
|
- Write the four quantum numbers for the differentiating electrons of li...
Text Solution
|
- Write four quantum numbers for 2p^(1) electron .
Text Solution
|
- How many electrons are occupied by an orbital ?
Text Solution
|
- Which rule is violated in the following electronic configuration?
Text Solution
|
- How many maximum number pf electrons can be accommodated in a principa...
Text Solution
|
- The maximum number of electrons that can be accommodated in the L-shel...
Text Solution
|
- How many maximum number of electrons can be accommodated in d sub-shel...
Text Solution
|
- How many sub-shells present in a 'M' principal energy shell?
Text Solution
|
- How many orientations can 's-orbital' exhibit ?
Text Solution
|
- What happens when an electron gains energy ?
Text Solution
|
- What happens when an object is suitably excited by heating ?
Text Solution
|
- Why do different elements emit different flame colours when heated by ...
Text Solution
|