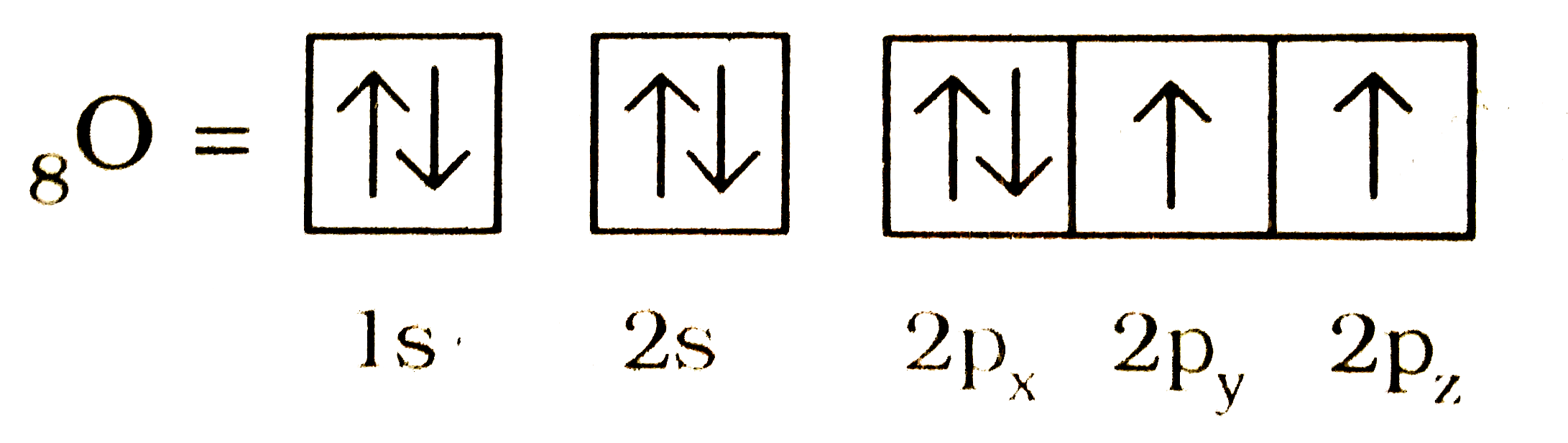Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - IV 4 MARKS )|32 VideosSTRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - II 1 MARK)|71 VideosREFLECTION AND REFRACTION
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|24 VideosSUMMATIVE ASSESSMENT
VGS PUBLICATION-BRILLIANT|Exercise SUMMATIVE ASSESSMENT|28 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-STRUCTURE OF ATOM -CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - III 2 MARKS QUESTIONS )
- Explain Hund's rule with an example .
Text Solution
|
- The electronic configuration of Sodium is 1s^(2) 2s^(2) 2p^(6) 3s^(1) ...
Text Solution
|
- Name the principle , which says an orbital can hold only 2 electrons a...
Text Solution
|
- For a better understanding about the electrode configuration in an ato...
Text Solution
|
- Write the electronic configuration of the atom of an element having at...
Text Solution
|
- Write the 'Octet Rule' . How does Mg (12) get stability while reacting...
Text Solution
|
- The electron enters into 4 s orbital after filling 3 p orbital but not...
Text Solution
|
- Write the electronic configuration of Na^(+) and Cl^(-)
Text Solution
|
- Observe the given table and answer the question Mention the dival...
Text Solution
|
- Observe the given table and answer the question Name the element ...
Text Solution
|
- Your friend is unable to understand n l^(x) . What questions will you ...
Text Solution
|
- Why do valency electrons involve in bond formation , than electrons of...
Text Solution
|
- What are characteristics of electromagnetic waves ?
Text Solution
|
- Write Planck's equation .
Text Solution
|
- Give the equation which give electromagnetic energy (light) that can h...
Text Solution
|
- Explain Pauli's Exclusion principle with an example.
Text Solution
|
- Explain Aufbau principle with an example .
Text Solution
|
- There is an electron in one atom with n = 1, l = O, m(l) = O. a) P...
Text Solution
|
- Guess the orbital. If 1) It's energy lies in between the energies o...
Text Solution
|
- The electronic configuration of an atom is as follows 1s^(2) 2s^(2) 2...
Text Solution
|