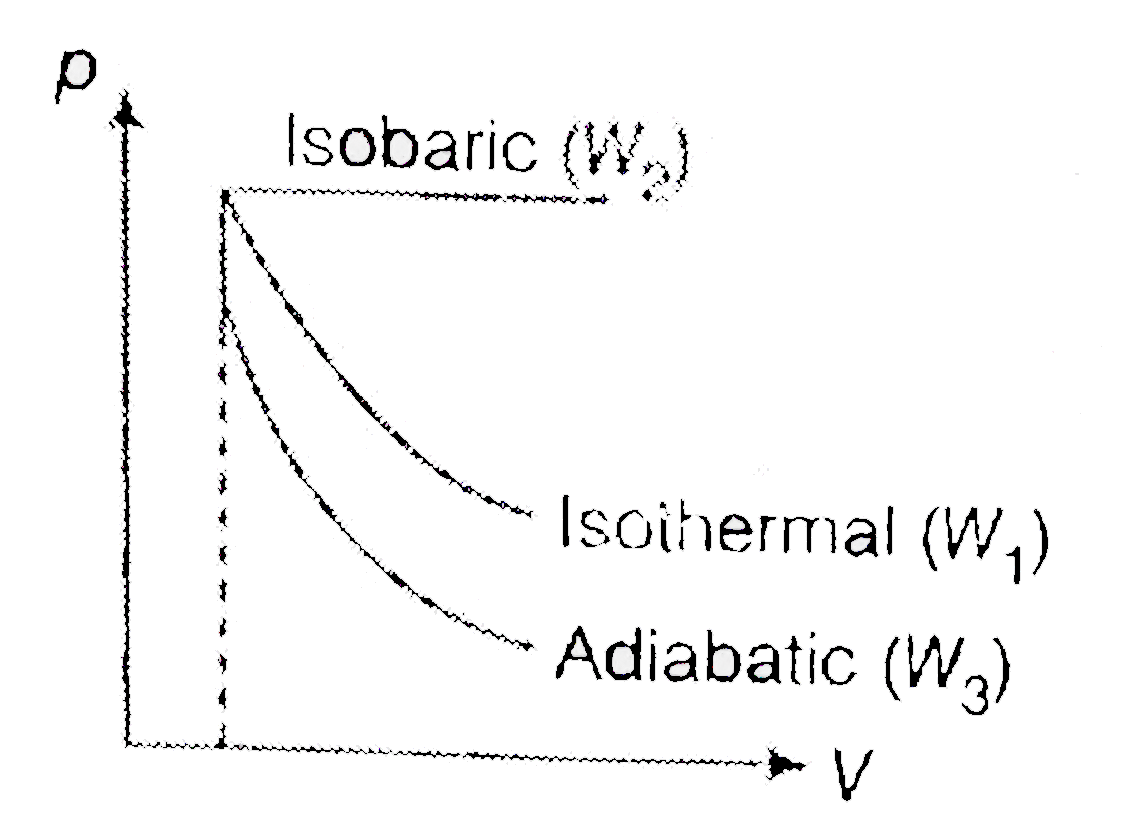A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LAWS OF THERMODYNAMICS
BITSAT GUIDE|Exercise 7|2 VideosLAWS OF THERMODYNAMICS
BITSAT GUIDE|Exercise 8|2 VideosLAWS OF THERMODYNAMICS
BITSAT GUIDE|Exercise 5|2 VideosHEAT, TEMPERATURE AND CALORIMETRY
BITSAT GUIDE|Exercise Bitsat archives|9 VideosMAGNETOSTATICS
BITSAT GUIDE|Exercise BITSAT Archives|8 Videos
Similar Questions
Explore conceptually related problems