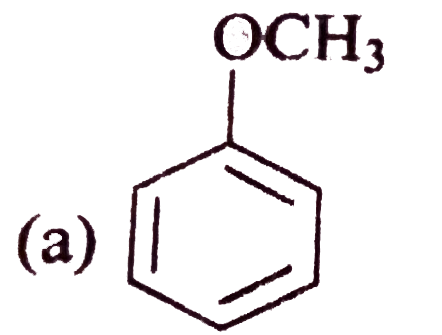
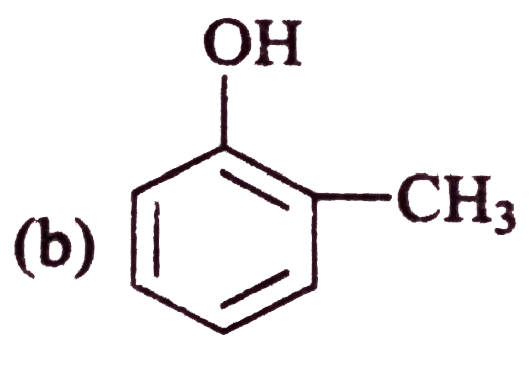
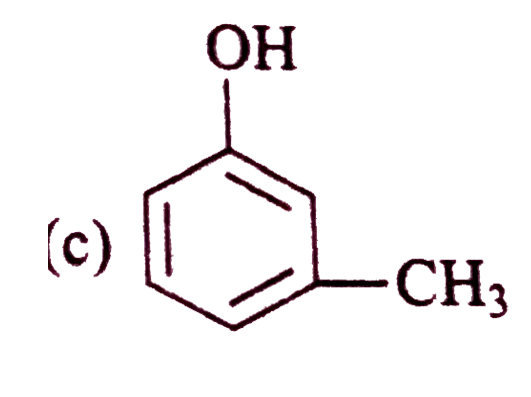
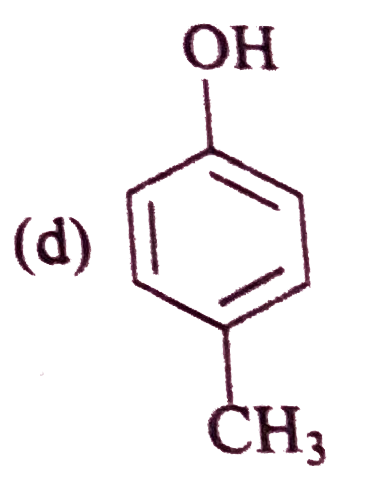
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS AND ETHERS
GRB PUBLICATION|Exercise level 59|1 VideosALCOHOLS AND ETHERS
GRB PUBLICATION|Exercise level 60|1 VideosALCOHOLS AND ETHERS
GRB PUBLICATION|Exercise level 57|1 VideosALCOHOL, ETHER AND EPOXY
GRB PUBLICATION|Exercise STRAIGHT OBJECTIVE TYPE|22 VideosBIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFE
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|40 Videos
Similar Questions
Explore conceptually related problems