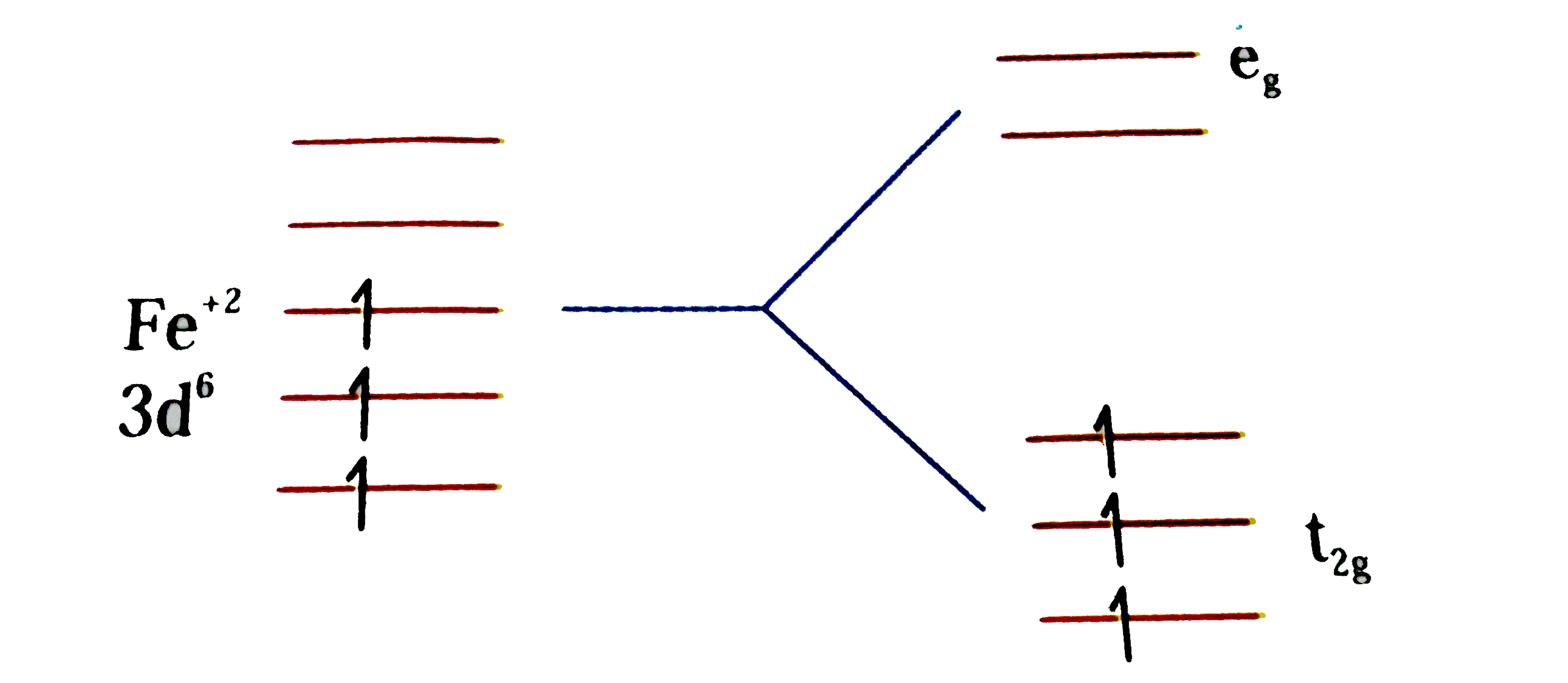
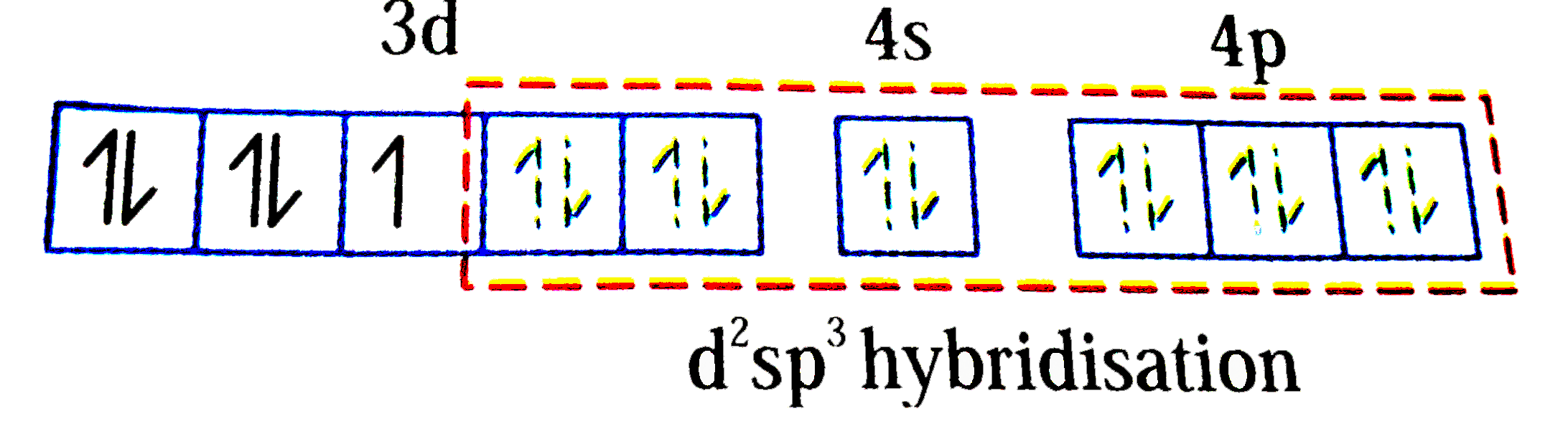
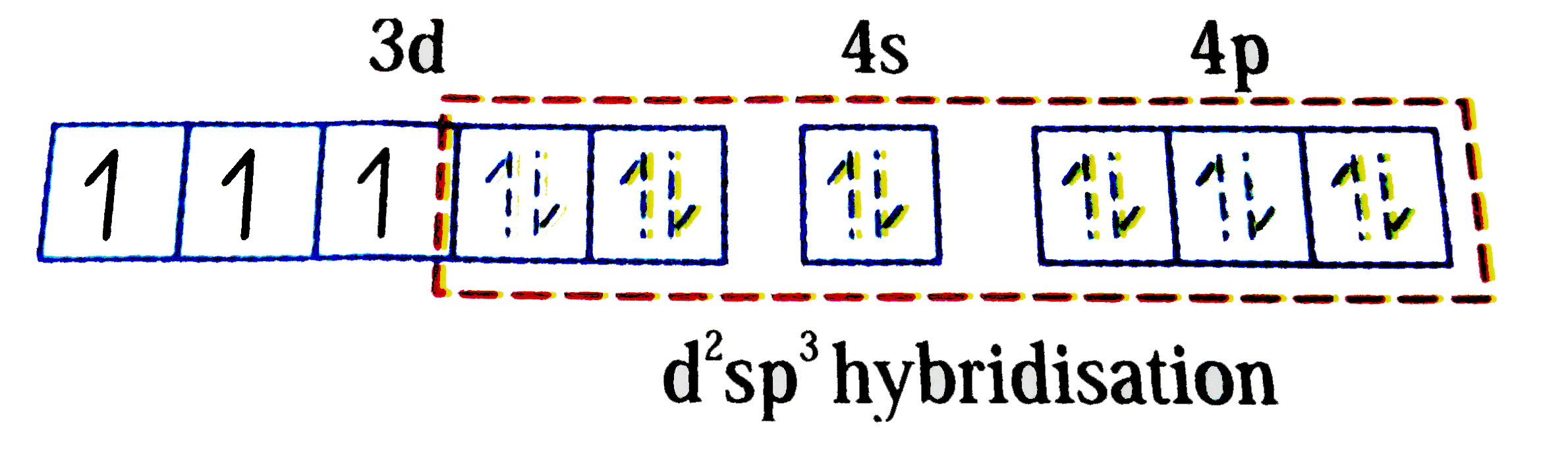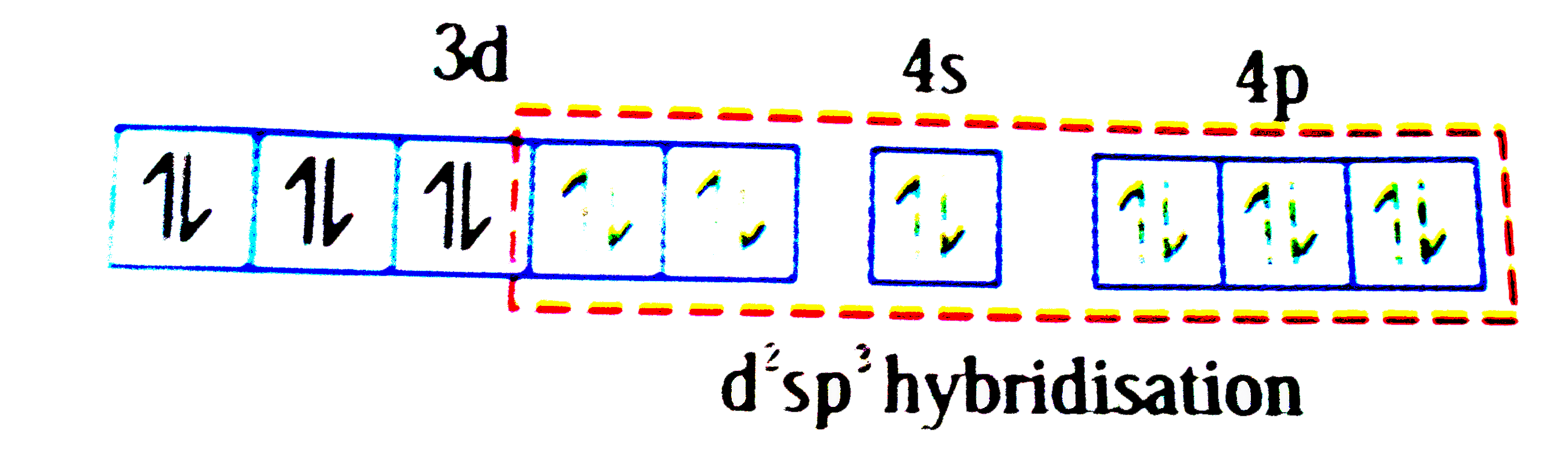A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPLEXES
NARAYNA|Exercise Comprehension-1|3 VideosCOORDINATION COMPLEXES
NARAYNA|Exercise Comprehension-2|3 VideosCOORDINATION COMPLEXES
NARAYNA|Exercise EXERCISE-8|6 VideosCO-ORDINATE COMPOUNDS
NARAYNA|Exercise Exercise-4|60 VideosD - BLOCK ELEMENTS
NARAYNA|Exercise CHECK YOUR GRASP|7 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-COORDINATION COMPLEXES-EXERCISE-9
- STATEMENT -1 A solution of [Ni(H(2)O)(6)]^(2+) is geen but a solution ...
Text Solution
|
- STATEMENT-1 The value of Delta(o) for M^(3+) complexes are always much...
Text Solution
|
- STATEMENT-1: All the complexes of Pt (+squaresquare) and Au (+squaresq...
Text Solution
|
- STATEMENT-1 : diamminedichloroplatinum (squaresquare) is more soluble ...
Text Solution
|
- STATEMENT-1 : [Co(NH(3))(4)Cl(2)]^(+) can exist in cis-and trans-forms...
Text Solution
|
- STATEMENT-1: In complex [Cr(NH(3))(4)BrCl]Cl, the'spin only' magnetic ...
Text Solution
|
- S-1 : [Cr(NH(3))(6)]^(3+) is a inner orbital complex with S-2: The c...
Text Solution
|
- s-1: [MnCl(6)]^(3),[FeF(6)]^(3-) and [CoF(6)]^(-3) are paramagnetic ha...
Text Solution
|
- S-1: The [Co(o x)(3)]^(3-) complex is diamagnetic andgains stability t...
Text Solution
|
- Consider the following satements : S(1): Generally square planare c...
Text Solution
|
- The ionisation isomer of [Cr(H(2)O)(4) Cl (NO(2))]Cl
Text Solution
|
- Consider the following statements and arrange in the order of the true...
Text Solution
|
- What is the coordination number of Co in [Co(NH(3))(4)(H(2)O)Br](NO(3)...
Text Solution
|