Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ANNUAL EXAMINATION QUESTION PAPER SOUTH-2017-PART-E
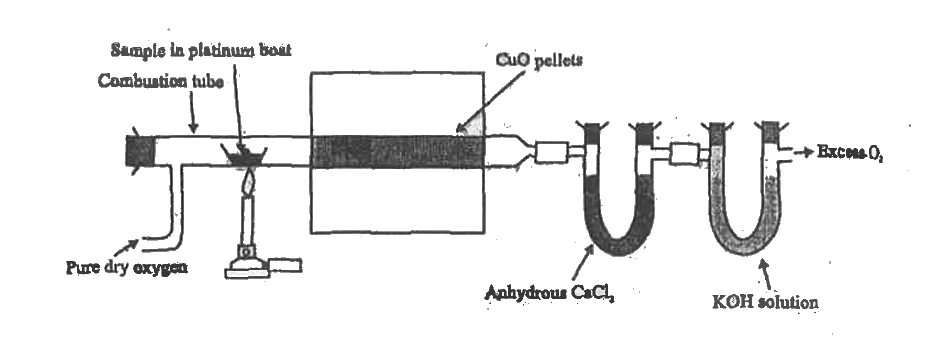
- Write the principle involved in the estimation of carbon and hydrogen....
Text Solution
|
- Write the resonance structure of benzene.
Text Solution
|
- How sulphur is estimated by Carius method and give calculation.
Text Solution
|
- Explain position isomerism with example.
Text Solution
|
- Explain the mechanism of nitration of benzene .
Text Solution
|
- Explain Wurtz reaction with example.
Text Solution
|