Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ELECTROCHEMISTRY-Problem Section
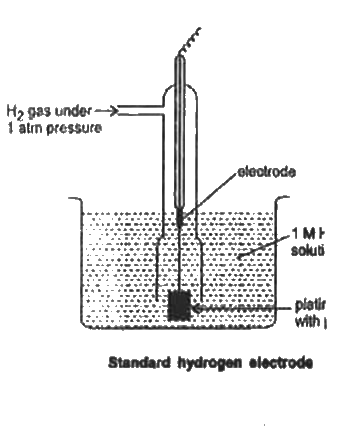
- Describe the construction and working of standard hydrogen electrode.
Text Solution
|
- E(cu)^(0)=0.34 and E(zn)^(0)=-0.76V. Calculate E("cell")^(0).
Text Solution
|
- E(Cu)^(0)=+0.34V and E(Ag)^(0)=+0.8V" calculate "E(cell")^(0).
Text Solution
|
- Calculate the EMF of the cell for the reaction. Mg((s))+2Ag((aq))^+ ...
Text Solution
|
- Calculate the e.m.f. of the cell in which the following reaction takes...
Text Solution
|
- Represent the cell in which the following reaction takes place Mg(s)...
Text Solution
|
- Calculate EMF of the cell represents below Zn//Zn^(2+)(C=0.1M)||Cu^(2+...
Text Solution
|
- For the standard cell Cu(s)|Cu^(2+)(aq)||Ag^(+)(aq)|Ag(s). [E((Cu^(2+)...
Text Solution
|
- For the standard cell Cu(s)|Cu^(2+)(aq)||Ag^(+)(aq)|Ag(s). [E((Cu^(2+)...
Text Solution
|
- Find the value of AG^(@) at 25^(@)C for the following electrochemical ...
Text Solution
|
- Calculate e.m.f. of cell for the reaction : Mg((s))+Cu^(2+)"(0.0001 ...
Text Solution
|
- Calculate DeltarG^@ for the following reactions: Fe^(+2) (aq)+Ag^(+)...
Text Solution
|
- Find the value of AG^(@) at 25^(@)C for the following electrochemical ...
Text Solution
|
- (a) The electrode potential for the Daniell cell given below is 1.1 V....
Text Solution
|
- Calculate standard free energy change for reaction Zn(S)+2Ag^(+)(aq) L...
Text Solution
|
- Calculate the equilibrium constant for the reaction Cu(s)+2Ag+(aq)ra...
Text Solution
|
- Calculate the equilibrium constant for the reaction Cu(s)+2Ag+(aq)ra...
Text Solution
|
- The cell in which of the following reaction occurs: 2Fe^(3+)(aq)+2I^...
Text Solution
|
- Resistance of a conductivity cell filled with 0.02 M KCl solution is 5...
Text Solution
|
- The resistance of solution of a salt occupying a volume between two pl...
Text Solution
|
- c) Resistance of a conductivity cell containing 0.1 M KCl solution is ...
Text Solution
|