Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-EXAM QUESTION PAPER JULY WITH ANSWER (2015)-PART E
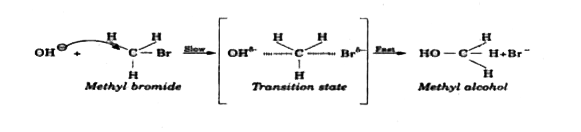
- Expain the SN^2 mechanism
Text Solution
|
- What is the reagent used in the conversion of alkyl halide into alkene...
Text Solution
|
- Complete the reaction. CH3-CH2-Br+underset("alco")AgCNunderset(/\)to
Text Solution
|
- What are enantiomers ?
Text Solution
|
- Explain the Kolbe's reaction.
Text Solution
|
- Complete the reactions : R-CH2-OHoverset(cu)underset(300^(@)C)to
Text Solution
|
- Complete the following reaction
Text Solution
|
- Write the general equation of Williamson's ether synthesis
Text Solution
|
- How would you prepare acetaldeyde from acetyl cholride. Name the react...
Text Solution
|
- Name the reagent used in the conversion of ketone to hydrocarbon. Name...
Text Solution
|
- Acetaldebydc does not undergo Cannizzaro's reaction, Why?
Text Solution
|
- Name the major product formed when nitrous acid is treated with Meth...
Text Solution
|
- Aniline at low temperature.
Text Solution
|
- Explain the Hoffmann's bromamide reaction.
Text Solution
|
- Write the IUPAC name of (CH(3))(2)N-CH(2)-CH(3).
Text Solution
|
- Give the Haworth structure of lactos.
Text Solution
|
- What are hormones ? Give an example.
Text Solution
|
- Which nitrogenous base present in DNA but not in RNA ?
Text Solution
|
- How is neoprene prepared ?
Text Solution
|
- What is bio-degradeable polymers ? Give example.
Text Solution
|