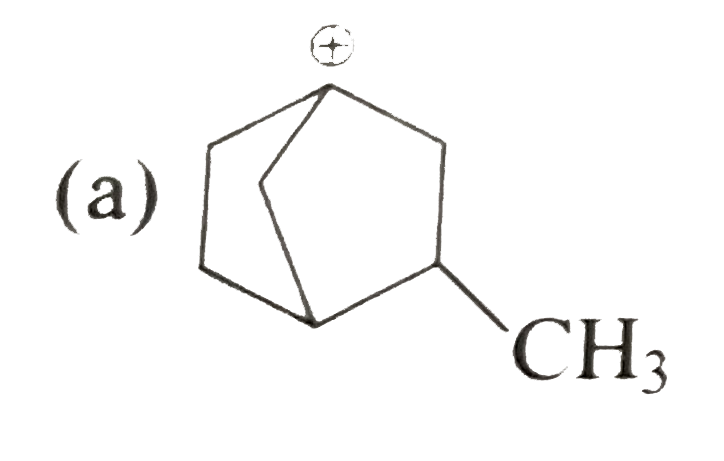
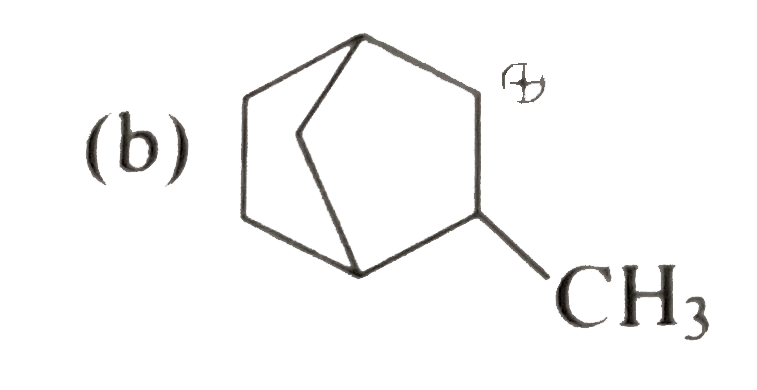
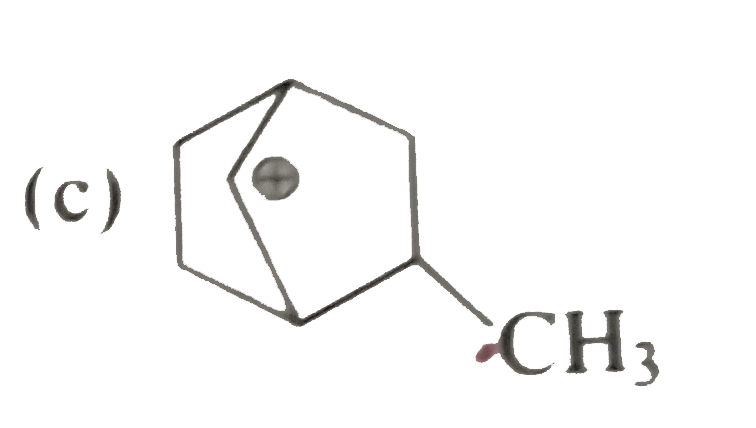
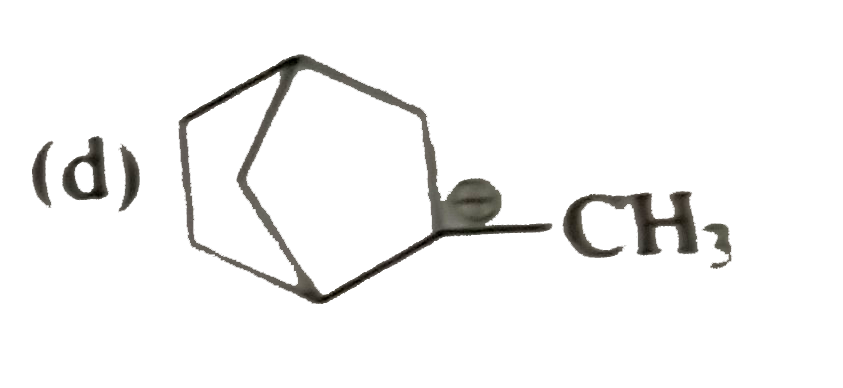
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.51 To Q.75)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.76 To Q.100)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.1 To Q.25)|25 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-GENERAL ORGANIC CHEMISTRY-Level 2 (Q.26 To Q.50)
- Acylium cation has two resonating sturctures (I) and (II), R-underse...
Text Solution
|
- Consider the following three amines, (1) CH(3)CH(2)-overset(* *)NH(2...
Text Solution
|
- The C-Cl bond length is shortest in :
Text Solution
|
- Which of the following pairs of structure are resonance structure?
Text Solution
|
- Which of the following is pair of resonance structure?
Text Solution
|
- The most stable diene is :
Text Solution
|
- Which of the following cation would have greatest stability?
Text Solution
|
- Which of the following is not valid resonance structre of polyene?
Text Solution
|
- Most contributing structure in introethene is :
Text Solution
|
- The most stable resonating structure of CH(3)-underset(* *)overset(* ...
Text Solution
|
- Which of the following does not represent structure of ?
Text Solution
|
- The correct stability order of following species is :
Text Solution
|
- Choose the correct staement :
Text Solution
|
- Which carbocation is most stabilised among following?
Text Solution
|
- Which of the following cations is most stable?
Text Solution
|
- Which of the following cations is most stable?
Text Solution
|
- Which of the following cations is most stable?
Text Solution
|
- Which of the folowing carbanions is most stable?
Text Solution
|
- In which of the following 2nd anion is more stable than first?
Text Solution
|
- Arrange the following carbanions in decreasing order of stability: C...
Text Solution
|