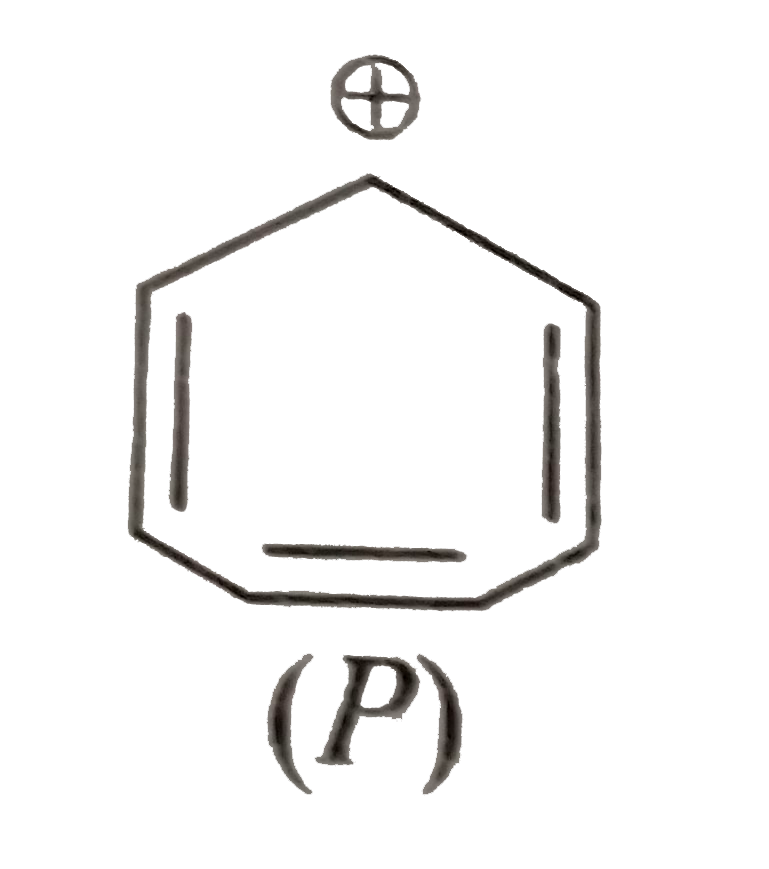
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.76 To Q.100)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.101 To Q.125)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.26 To Q.50)|25 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-GENERAL ORGANIC CHEMISTRY-Level 2 (Q.51 To Q.75)
- Arrage the following cations in decreasing order of stability: CH...
Text Solution
|
- Among the following which is most stabilised cation ?
Text Solution
|
- Which of the following anions is most stabilised?
Text Solution
|
- Arrange the following anions in decreasing order of stability : H-ov...
Text Solution
|
- Which of the following is most stabilised by hyperconjugation?
Text Solution
|
- rArrThe decreasing order of stability of following cation is (Question...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- rArr The decreasing order of stability of following anions is (Questio...
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- The most unlikely representation of resonance structure of p-nitrophen...
Text Solution
|
- Which will be MOST stable resonationg structure?
Text Solution
|
- Which of the following acids has lowest value of dissociation canstant...
Text Solution
|
- Arrage the following acids in decreasing order of acidity : H-unders...
Text Solution
|
- Which of the following acids have the lowest pK(a) value :-
Text Solution
|